Fluorescence Digital Image Gallery
Human Bone Osteosarcoma Cells (U-2 OS Line)
The U-2 OS cell line, originally known as the 2T line, was derived from the bone tissue of a fifteen-year-old human female suffering from osteosarcoma. Established by J. Ponten and E. Saksela in 1964, the original cells were taken from a moderately differentiated sarcoma of the tibia.
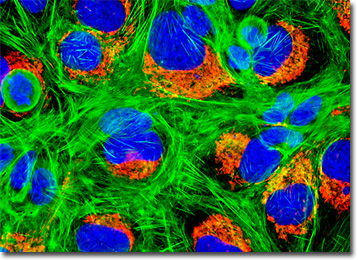
U-2 OS cells exhibit typical epithelial morphology and viruses were not detected in the line during co-cultivation with WI-38 cells or in CF tests against simian virus 40 (SV40), respiratory syncytial virus (RSV), or adenoviruses. Mycoplasma contamination in major stocks of the U-2 OS line was detected and subsequently eliminated in 1972. Cells are positive for insulin-like growth factor I (IGF-I) and insulin-like growth factor II (IGF II) receptors and express a number of antigens, including blood type A, Rh+, HLA A2, Aw30, B12, Bw35, and B40(+/-).
Osteosarcoma is the most common type of bone cancer in the world and is the sixth most frequently occurring cancer in children. Since this kind of tumor usually develops from osteoblasts, which are the cells from which bone develops, osteosarcoma most often affects teenagers who are undergoing the characteristic rapid bone growth of adolescence. Moreover, although the U-2 OS cell line was derived from a female, boys, who typically grow more than girls, are about twice as likely to experience the disease. In most cases of osteosarcoma, no specific cause or predisposing risk factor can be identified. Sometimes, however, rare genetic conditions are believed to have played a part in osteosarcoma development. The most widely recognized risk factor for the disease is the presence of a gene that is also responsible for hereditary retinoblastoma, a tumor of the eye that occurs primarily in early childhood.
The culture of U-2 OS cells presented in the digital image above was transfected with a pDsRed-Mitochondria plasmid subcellular localization vector, thus localizing a red fluorescent protein tag to the intracellular mitochondrial network. Stable transfectants were isolated, grown in monolayer culture, and then fixed, permeabilized, and labeled with a combination of DAPI and Alexa Fluor 488 conjugated to phalloidin, targeting DNA and filamentous actin, respectively. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles.
Additional Fluorescence Images of Human Bone Osteosarcoma (U-2 OS) Cells
Histone and Peroxisome Distribution in U-2 OS Cells - Nuclear histone proteins were targeted in a culture of human osteosarcoma cells with mouse anti-histone (pan) monoclonal antibodies, which were imaged with goat anti-mouse Fab fragments conjugated to the cyanine dye, Cy2 (labeling the nucleus). The specimen was simultaneously labeled for peroxisomes with Rhodamine Red conjugated to goat secondary antibodies that target rabbit anti-PMP 70 (peroxisomal membrane protein 70). Alexa Fluor 350 conjugated to phalloidin was utilized to counterstain the cytoskeletal F-actin network.
Human Osteosarcoma Cells with MitoTracker CMXRos, Alexa Fluor 488, and DAPI - A healthy culture of U-2 OS human cancer cells was stained for mitochondria with MitoTracker CMXRos, a derivative of X-rosamine. After fixation and permeabilization, the cells were labeled with Alexa Fluor 488 conjugated to phalloidin and DAPI, targeting filamentous actin and nuclear DNA, respectively.
Golgi Network and Filamentous Actin in U-2 OS Cell Cultures - The adherent culture of human osteosarcoma (U-2 OS) cells illustrated in this section was stained with Oregon Green 488 conjugated to wheat germ agglutinin, a plant-derived lectin that targets the Golgi apparatus, as well as Alexa Fluor 568 conjugated to phalloidin for cytoskeletal actin. Nuclei were labeled with the ultraviolet-absorbing probe DAPI.
U-2 OS Human Osteosarcoma Cells with the Cyanines Cy2 and Cy3, as well as Alexa Fluor 350 - Histones present in the nuclei of cancerous bone cells (U-2 OS line) were immunofluorescently labeled with primary anti-histone mouse monoclonal antibodies followed by goat anti-mouse Fab heavy and light chain fragments conjugated to Cy3. In addition, the specimen was simultaneously stained for filamentous actin with Alexa Fluor 350 conjugated to phalloidin, and for peroxisomes with Cy2 conjugated to goat secondary antibodies that target rabbit anti-PMP 70 (peroxisomal membrane protein).
Subcellular Localization of Mitochondria in U-2 OS Cells with DsRed Fluorescent Protein - A culture of human osteosarcoma cells was transfected with a pDsRed-Mitochondria plasmid subcellular localization vector, thus localizing a red fluorescent protein tag to the intracellular mitochondrial network. After isolation of stable transfectants, a healthy culture was fixed and subsequently labeled with DAPI and Alexa Fluor 488 conjugated to phalloidin, targeting DNA and F-actin, respectively.
Targeting the Golgi Complex in Cancerous Human Bone Cells with Lectins - In this section, the featured culture of human osteosarcoma (U-2 OS) cells was labeled with Oregon Green conjugated to lectin GS-II, which is isolated from the seeds of a tropical legume and often utilized as a selective stain for the Golgi apparatus. The culture was additionally labeled for the cytoskeletal filamentous actin network with Alexa Fluor 568 conjugated to phalloidin, and for nuclear DNA with the ultraviolet-absorbing probe DAPI.
Distribution of the Mitochondrial and Filamentous Actin Networks in U-2 OS Cells - The mitochondria present in a culture of U-2 OS human cancer cells were targeted with MitoTracker Red CMXRos, a derivative of X-rosamine. In addition, the culture was labeled for F-actin and nuclear DNA with Alexa Fluor 488 conjugated to phalloidin and DAPI, respectively.
DsRed Fluorescent Protein Subcellular Organelle Localization in Human Osteosarcoma Cells - A stable line of U-2 OS transfectants containing a subcellular mitochondrial localization vector of DsRed fluorescent protein fused to the mitochondrial targeting sequence of human cytochrome C oxidase were fixed, permeabilized, and blocked with bovine serum albumen before being labeled with Alexa Fluor 350 conjugated to phalloidin. The nuclei were counterstained with SYTOX Green.
Human Osteosarcoma Cells with Alexa Fluor 488, DsRed Fluorescent Protein, and DAPI - A culture of U-2 OS human cancer cells was transfected with a recombinant plasmid vector containing a chimeric fusion gene product of DsRed fluorescent protein and the mitochondrial targeting sequence from subunit VIII of human cytochrome C oxidase. Stable transfectants were fixed, permeabilized, and treated with phalloidin conjugated to Alexa Fluor 488 before being counterstained with DAPI.
Visualizing the Golgi Complex in U-2 OS Cancer Cells - The U-2 OS cell culture featured in this section was fixed, permeabilized, washed, and blocked with 10-percent normal goat serum in phosphate-buffered saline prior to immunofluorescent labeling with rabbit primary antibodies to giantin, a protein resident in the Golgi complex of mammalian cells. The culture was subsequently stained with a mixture of secondary antibodies conjugated to Alexa Fluor 488. In addition, histones were immunofluorescently labeled with primary anti-histone mouse monoclonal antibodies followed by goat anti-mouse Fab heavy and light chain fragments conjugated to Texas Red. The filamentous actin network was counterstained with Alexa Fluor 350 conjugated to phalloidin.
Distribution of the Filamentous Actin Network and Golgi Bodies in Human Osteosarcoma Cells - A culture of human osteosarcoma (U-2 OS) cells was stained with Alexa Fluor 594 conjugated to phalloidin and Hoechst 33342, targeting the cytoskeletal filamentous actin network and nuclear DNA, respectively. The cells were also immunofluorescently labeled with primary anti-giantin rabbit monoclonal antibodies followed by goat anti-rabbit secondary antibodies conjugated to Alexa Fluor 488 in order to target the Golgi apparatus.
Targeting the Endoplasmic Reticulum in U-2 0S Cultures with Immunofluorescence - An adherent culture of human osteosarcoma cells was immunofluorescently labeled with primary anti-calreticulin rabbit monoclonal antibodies followed by goat anti-rabbit secondary antibodies conjugated to Cy3, targeting the endoplasmic reticulum. In addition, the cells were stained with Alexa Fluor 488 conjugated to phalloidin and Hoechst 33342, which bind respectively with filamentous actin and nuclear DNA.
U-2 OS Cells with Rhodamine Red-X, Cy2, and Alexa Fluor 350 - In a double immunofluorescence labeling experiment, a culture of U-2 OS cells was treated with a cocktail of mouse anti-histones (pan) and rabbit anti-PMP 70 (peroxisomal membrane protein) primary antibodies. The target proteins were subsequently visualized with goat anti-mouse and anti-rabbit secondary antibodies conjugated to Cy2 and Rhodamine Red, respectively. The filamentous actin cytoskeletal network was counterstained with Alexa Fluor 350 conjugated to phalloidin.
Adhesion Junctions in Human Osteosarcoma Cells - An adherent culture of human osteosarcoma cells was immunofluorescently labeled with primary anti-vinculin mouse monoclonal antibodies followed by goat anti-mouse Fab heavy and light chain fragments conjugated to Cy2. In addition, the specimen was simultaneously stained for DNA with the ultraviolet-absorbing probe DAPI, and for the cytoskeletal filamentous actin network with Alexa Fluor 568 conjugated to phalloidin.
Mitochondria and F-Actin Distribution in Bone Cancer Cells - The human bone cancer (U-2 OS) cell culture featured in this section was labeled for mitochondria and the cytoskeletal F-actin network with MitoTracker Red CMXRos and Alexa Fluor 488 conjugated to phalloidin, respectively. Nuclei were counterstained with the ultraviolet-absorbing probe DAPI.
U-2 OS Bone Cancer Cells with Alexa Fluor 568, Alexa Fluor 488, and Hoechst 33342 - A healthy log-phase culture of human osteosarcoma cells was fixed, permeabilized, and blocked with 10-percent normal goat serum in phosphate-buffered saline prior to immunofluorescent labeling with rabbit primary antibodies to giantin. The culture was subsequently stained with a mixture of goat anti-rabbit secondary antibodies conjugated to Alexa Fluor 488, in order to visualize the giantin, and Alexa Fluor 568 conjugated to phalloidin for the filamentous actin cytoskeleton. Hoechst 33342 was used to visualize the nuclei.
U-2 OS Human Osteosarcoma Cells with MitoTracker Red CMXRos, Alexa Fluor 488, and DAPI - In what has now become a traditional fluorescence staining cocktail, the culture of osteosarcoma cells appearing in this section was stained with MitoTracker Red CMXRos, Alexa Fluor 488 conjugated to phalloidin, and DAPI, in order to target the mitochondrial network, filamentous actin, and nuclear DNA, respectively.
Immunofluorescence Targeting of Histones and Peroxisomes in Human Osteosarcoma Cell Cultures - In a double immunofluorescence labeling protocol, a culture of human bone cancer (U-2 OS) cells was treated with a cocktail of mouse anti-histone (pan) and rabbit anti-PMP 70 (peroxisomal membrane protein) primary antibodies. The target proteins were subsequently visualized with goat anti-mouse and anti-rabbit secondary antibodies conjugated to Texas Red and Alexa Fluor 488, respectively. The filamentous actin cytoskeletal network was counterstained with Alexa Fluor 350 conjugated to phalloidin, a bicyclic peptide isolated from the death cap mushroom (Amanita phalloides).
Enhanced Yellow Fluorescent Protein (EYFP) Subcellular Localization in U-2 OS Cells - An adherent culture of U-2 OS cells was transfected with a pEYFP-Mitochondria plasmid subcellular localization vector, which contains the mitochondrial targeting sequence from subunit VIII of human cytochrome C oxidase. The enhanced yellow fluorescent protein gene employed with this culture features several important amino acid substitutions that shift the emission maximum of green fluorescent protein (GFP) by approximately 18 nanometers, from 509 to 527 nanometers. The cells were additionally labeled with Alexa Fluor 568 conjugated to phalloidin and Hoechst 33258, targeting the filamentous actin network and nuclei, respectively.
Human Osteosarcoma (U-2 OS) Cells with Cy3, Alexa Fluor 488, and DAPI - The culture of human osteosarcoma cells featured in this section was immunofluorescently labeled with primary anti-vinculin mouse monoclonal antibodies followed by goat anti-mouse Fab heavy and light chain fragments conjugated to Cy3. Vinculin is a protein associated with focal adhesion and adherens junctions, which are membrane-associated complexes that serve as nucleation sites for actin filaments and as crosslinkers between the external medium, plasma membrane, and actin cytoskeleton. The cells were also stained with the DNA-specific fluorophore DAPI and an Alexa Fluor 488 phalloidin conjugate in order to target cytoskeletal F-actin.
Visualizing the Mitochondrial and Filamentous Actin Networks in Human Osteosarcoma Cells - Stable U-2 OS transfectants containing a subcellular mitochondrial localization vector of DsRed fluorescent protein fused to the mitochondrial targeting sequence of human cytochrome C oxidase were fixed, permeabilized, and blocked with bovine serum albumen before being labeled with Alexa Fluor 350 conjugated to phalloidin. The nuclei were counterstained with SYTOX Green.
BACK TO THE CULTURED CELLS FLUORESCENCE GALLERY
BACK TO THE FLUORESCENCE GALLERY
