Fluorescence Digital Image Gallery
Human Cervical Adenocarcinoma Cells (HeLa Line)
The HeLa line is one of the best-known cell lines in the world. Derived in 1951 from an adenocarcinoma of the cervix found in a 31-year-old woman (Henrietta Lacks), HeLa cells were the first human cells to survive indefinitely in the laboratory. The cells exhibit epithelial morphology and grow adherently, reproducing an entire generation about every 24 hours.
Cellular products of the HeLa line include keratin and lysophosphatidylcholine (lyso-PC), which induces AP-1 activity and c-jun N-terminal kinase activity (JNK1) by a protein kinase C-independent pathway. HeLa cells have been reported to contain human papilloma virus 18 (HPV-18) sequences. P53 expression in the cells has been described as low, though levels of pRB (retinoblastoma suppressor) are apparently normal.
The seemingly immortal nature of HeLa cells enabled researchers to carry out experiments that had been previously impossible and, consequently, they became a standard cell line soon after their discovery. HeLa cells have since been used to search for a cure for leukemia and the cause of cancer and to study genetic control mechanisms, protein synthesis, and cellular effects of radiation. Particularly notable is the crucial role the cells played in the development of the polio vaccine. HeLa cells first made it possible to grow the virus in a laboratory, a vital step towards being able to find a way to inhibit its proliferation. The cells were also used to help distinguish between strains of polio and determine which were responsible for the crippling effects of the disease. Then, when a vaccine was created, it was tested upon HeLa cells before being administered to humans.
Despite the many benefits HeLa cells have brought to the scientific realm, the extremely virulent cells have also been the cause of a significant amount of uncertainty and concern. Capable of reproducing an entire generation every 24 hours, by the late 1960s HeLa cells had invaded many other cell lines used in research. The problem went on without the knowledge of the scientists involved for many years. In 1974, however, California researcher Walter Nelson-Rees published a list of scientific studies tainted by the invasion of HeLa cells, ultimately discrediting millions of dollars of research.
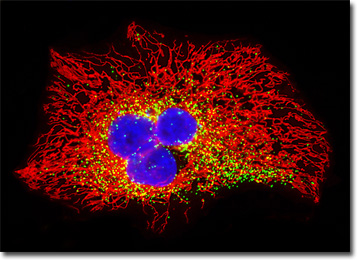
The HeLa carcinoma cell culture featured in the digital image above was transfected with an EGFP-peroxisomal targeting signal 1 (PTS1) fusion protein and stained with MitoTracker Red CMXRos. These fluorescent probes target the peroxisomes and intracellular microtubular network, respectively. The visible light absorption maximum of the EGFP-PTS1 chimera is 488 nanometers and the emission maximum occurs at 507 nanometers. In addition, the specimen was simultaneously stained with Hoechst 33342 (targeting the DNA in the nucleus; blue emission). Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles.
Additional Fluorescence Images of Human Cervical Adenocarcinoma (HeLa) Cells
Golgi Network in Cervical Adenocarcinoma Cells - A culture of HeLa carcinoma cells was immunofluorescently labeled with anti-human golgin-97 mouse monoclonal primary antibodies followed by goat anti-mouse Fab fragments conjugated to Alexa Fluor 488, in order to target the Golgi apparatus. In addition, the cells were labeled for filamentous actin with Alexa Fluor 568 conjugated to phalloidin, and for nuclear DNA with the ultraviolet-absorbing probe DAPI.
HeLa Cells with Enhanced Yellow Fluorescent Protein (EYFP) - In this section, the digital image presented features a culture of HeLa cells that was transfected with a pEYFP-Mitochondria plasmid subcellular localization vector, which contains the mitochondrial targeting sequence from subunit VIII of human cytochrome C oxidase. The enhanced yellow fluorescent protein gene used with this culture features several important amino acid substitutions that shift the emission maximum of green fluorescent protein (GFP) by approximately 18 nanometers, from 509 to 527 nanometers. The cells were additionally labeled with the nucleic acid stain SYTOX Orange and Alexa Fluor 350 conjugated to phalloidin, targeting DNA and filamentous actin, respectively.
Tubulin, F-Actin, and DNA Distribution in Carcinoma Cells - Human cervical adenocarcinoma cells (HeLa) were immunofluorescently labeled with anti-tubulin primary mouse monoclonal antibodies followed by goat anti-mouse Fab fragments conjugated to the cyanine dye, Cy2. The culture was counterstained for DNA with Hoechst 33258, and for the cytoskeletal F-actin network with Alexa Fluor 568 conjugated to phalloidin.
EGFP-Nucleus Subcellular Localization in Transfected HeLa Cells - A culture of HeLa carcinoma cells was transfected with a pEGFP-Nucleus plasmid subcellular localization vector. Plasmid pEYFP-Nucleus vector gene product expression in various cell types occurs due to the efficient intracellular translation of a fusion nucleotide sequence combining the enhanced green fluorescent protein domain with three tandem repeats of the nuclear localization signal (NLS) from simian virus 40 (SV40) large T-antigen. In addition, the cells were labeled with Alexa Fluor 568 conjugated to phalloidin in order to target the cytoskeletal filamentous actin network.
Adhesion Junctions in HeLa Cells - An adherent culture of the HeLa cervical carcinoma epithelial cells was immunofluorescently labeled with anti-vinculin mouse monoclonal primary antibodies followed by goat anti-mouse Fab heavy and light chain fragments conjugated to Cy3 (red emission). In addition, the specimen was simultaneously stained for DNA with the ultraviolet-absorbing probe Hoechst 33342, and for the cytoskeletal filamentous actin network with Alexa Fluor 488 conjugated to phalloidin.
Transfected HeLa Cells with MitoTracker Red CMXRos and Enhanced Green Fluorescent Protein Localized to the Peroxisomes - HeLa cervical carcinoma epithelial cells were transfected with an EGFP vector containing the peroxisomal targeting signal 1 (PTS1) fusion protein. Stable transfectants were isolated and subsequently labeled with MitoTracker Red CMXRos before being counterstained with Hoechst 33342. The specimen was imaged using a scanning confocal microscope equipped with an argon-ion and a helium-neon laser (488 and 543 nanometer excitation wavelengths, respectively).
Distribution of Histones, Peroxisomes, and Filamentous Actin in HeLa Cell Cultures - In a double immunofluorescence labeling experiment, an adherent culture of HeLa cells was treated with a cocktail of mouse anti-histones (pan) and rabbit anti-PMP 70 (peroxisomal membrane protein) primary antibodies, followed by goat anti-mouse and anti-rabbit secondary antibodies conjugated to Alexa Fluor 568 and Alexa Fluor 488, respectively, to target the nuclear histone proteins and peroxisomes. The filamentous actin network was imaged with Alexa Fluor 350 conjugated to phalloidin.
BACK TO THE CULTURED CELLS FLUORESCENCE GALLERY
BACK TO THE FLUORESCENCE GALLERY
