Fluorescence Digital Image Gallery
Embryonic Rat Thoracic Aorta Medial Layer Myoblast Cells (A-10 Line)
The clonal cell line A-10 was derived by B. Kimes and B. Brandt from the thoracic aorta of an embryonic rat (Rattus norvegicus) from the established strain DB1X. The thoracic aorta is a branch of the descending aorta, which transports blood from the heart to the other organs and parts of the body. This arbitrary anatomic entity is generally considered to extend from the arch of the aorta to the diaphragm.
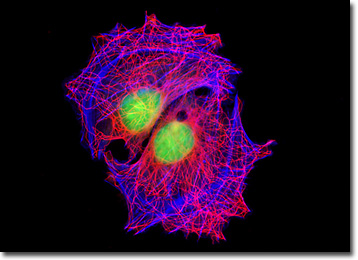
The cells grow adherently and exhibit myoblast morphology, possessing many of the properties characteristic of smooth muscle cells. Cellular products include myokinase, creatine phosphokinase, and myosin. A-10 cells produce spontaneous action potentials at the stationary phase of the growth cycle and exhibit an increase in activity of the enzymes myokinase and creatine phosphokinase.
A-10 cells are often utilized in medical research because smooth muscle cells are sometimes implicated in the development of various diseases and conditions. For example, an increase in smooth muscle cell size and the number of cells present in a given location have been suggested as a possible causes of hypertension, better known as high-blood pressure. According to this theory, the extra smooth muscle in the arterial walls could result in a corresponding amplification of the constrictive capacity of the artery as well as increase the thickness of the wall of the blood vessel. Both of these events could effectively constrict the artery's lumen and subsequently decrease the amount of blood flow. To counteract such an increased resistance to the flow of blood, the cardiovascular system would likely respond by raising blood pressure in order to ensure that sufficient supplies of blood were capable of reaching the many tissues of the body.
The rat thoracic aorta cells presented in the digital image above were immunofluorescently labeled with mouse anti-alpha-tubulin primary antibodies followed by goat anti-mouse secondary antibodies conjugated to Alexa Fluor 568. In addition, the specimen was stained with Alexa Fluor 350 conjugated to phalloidin and SYTOX Green, targeting the filamentous actin network and nuclei, respectively. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles.
Additional Fluorescence Images of Rat Thoracic Aorta (A-10) Cells
Rat Thoracic Aorta Cell with Alexa Fluor 488, Texas Red, and DAPI - A culture of rat thoracic aorta (A-10) cells was labeled with the enzyme DNase I conjugated to the fluorochrome Texas Red in order to reveal unpolymerized globular (G) actin, which accumulates in the center of the cell. In addition, the specimen was simultaneously stained for DNA with the ultraviolet-absorbing probe DAPI, and for the cytoskeletal filamentous actin network with Alexa Fluor 488 conjugated to phalloidin.
Enhanced Green Fluorescent Protein in Transfected A-10 Cells - Rat thoracic aorta (A-10) cells were transfected with a chimeric EGFP plasmid vector that expresses a fluorescent fusion protein targeted at cytoplasmic actin. The fusion protein is incorporated into the growing actin filament network to enable visualization of actin-containing subcellular structures in living and fixed cells. The specimen illustrated in this section was also stained for mitochondria with MitoTracker Red CMXRos, and for DNA in the nucleus with the ultraviolet-absorbing probe DAPI.
A-10 Cells with MitoTracker Red CMXRos, Alexa Fluor 350, and SYTOX Green - In a manner similar to the cells illustrated above, this adherent culture was fluorescently labeled with MitoTracker Red CMXRos, Alexa Fluor 350 conjugated to phalloidin, and SYTOX Green, targeting the mitochondria, filamentous actin network, and nuclei, respectively. The bright green nuclei can be visualized along with a cluster of red mitochondria and light blue actin stress fibers.
Focal Adhesion Sites in Rat Thoracic Aorta Cells - A culture of A-10 cells was immunofluorescently labeled with primary anti-vinculin mouse monoclonal antibodies followed by goat anti-mouse Fab fragments conjugated to Cy3 (red fluorescence emission). Note the prominent staining of the cellular attachment network in the central portion and periphery of these cells. In addition, the specimen was simultaneously stained for DNA with the ultraviolet-absorbing probe DAPI, and for the cytoskeletal filamentous actin network with Alexa Fluor 488 conjugated to phalloidin.
The Golgi Complex in A-10 Cell Cultures - A culture of rat thoracic aorta cells was fixed, permeabilized, and blocked with 10-percent normal goat serum in phosphate-buffered saline prior to immunofluorescent labeling with rabbit primary antibodies to giantin, a protein resident in the Golgi complex of mammalian cells. The culture was subsequently stained with a mixture of goat anti-rabbit secondary antibody fragments (heavy and light chain) conjugated to Cy2. In addition, the cells were labeled with Alexa Fluor 568 conjugated to phalloidin (targeting the filamentous actin network), and for DNA with Hoechst 33342.
A-10 Cells with MitoTracker Red CMXRos, Alexa Fluor 488, and Hoechst 33258 - In what has become a standard fluorescence staining protocol, an adherent culture of rat thoracic aorta (A-10) cells was labeled with MitoTracker Red CMXRos before fixing, and the cells were subsequently stained with Alexa Fluor 488 conjugated to phalloidin, followed by Hoechst 33258, a popular DNA-binding counterstain.
Rat Thoracic Aorta Cells with Alexa Fluor 350 - A third adherent culture of A-10 cells was fluorescently labeled with MitoTracker Red CMXRos, Alexa Fluor 350 conjugated to phalloidin, and SYTOX Green, targeting the mitochondria, filamentous actin network, and nuclei, respectively. Similar to the other images presented with this staining regimen, the cellular nuclei appear bright green, while the mitochondria are red and the actin stress fibers are deep blue.
Vinculin Distribution in Rat Thoracic Aorta Cells - An adherent culture of rat aorta cells was immunofluorescently labeled with primary anti-vinculin mouse monoclonal antibodies followed by goat anti-mouse Fab heavy and light chain fragments conjugated to Cy3 (red fluorescence emission). In addition, the specimen was simultaneously stained for DNA with the ultraviolet-absorbing probe DAPI, and for the cytoskeletal filamentous actin network with Alexa Fluor 488 conjugated to phalloidin.
Immunofluorescence Labeling of beta-Catenin and Histones in Rat Thoracic Aorta Cells - The technique of double immunofluorescence was employed to simultaneously label an adherent culture of rat thoracic aorta (A-10 line) cells with mouse anti-histone and rabbit anti-beta-catenin primary antibodies, followed by goat anti-mouse and anti-rabbit secondary antibodies conjugated to Marina Blue (histones) and Rhodamine Red-X (beta-catenin), respectively. The culture was counterstained with Alexa Fluor 488 conjugated to phalloidin, targeting the intracellular filamentous actin network.
Mitochondrial and Actin Distribution in A-10 Cell Cultures - A culture of adherent A-10 rat thoracic aorta cells was fluorescently triple-labeled with MitoTracker Red CMXRos, Alexa Fluor 350 conjugated to phalloidin, and SYTOX Green, targeting the mitochondria, filamentous actin network, and nuclei, respectively. In this image, the bright red mitochondrial network is superimposed on a deep blue actin cytoskeletal framework centered around the green nuclei.
Rat Thoracic Aorta Medial Layer Myoblast Cells with Texas Red-X, Oregon Green 488, and Hoechst 33258 - In order to label the intermediate filaments in a log phase adherent A-10 culture, the fixed and permeabilized cells were blocked and treated with mouse anti-vimentin (porcine eye lens) primary antibodies followed by goat anti-mouse secondary antibodies (IgG) conjugated to Texas Red-X. Filamentous actin was visualized with phalloidin conjugated to Oregon Green 488, while the nuclei were stained with Hoechst 33258.
Triple-Fluorophore Labeling of Rat Thoracic Aorta Myoblast Cell Cultures - Using a popular fluorophore combination, a log phase monolayer culture of A-10 cells was treated with MitoTracker Red CMXRos for one hour, washed and fixed in growth medium containing paraformaldehyde, permeabilized, and blocked with bovine serum albumen. The culture was subsequently stained with Alexa Fluor 488 conjugated to phalloidin and counterstained with Hoechst 33258.
The Rat Thoracic Aorta Cytoskeletal Tubulin and Actin Networks - Rat thoracic aorta cells in culture were immunofluorescently labeled with mouse anti-alpha-tubulin primary antibodies followed by goat anti-mouse secondary antibodies conjugated to Alexa Fluor 568. In addition, the specimen was stained with Alexa Fluor 350 conjugated to phalloidin and SYTOX Green, targeting the filamentous actin network and nuclei, respectively.
Visualizing the Nuclear Pore Complex in Rat Thoracic Aorta Cell Cultures - Using a monoclonal antibody directed against the nuclear pore complex proteins, an adherent culture of rat thoracic aorta (A-10) cells was fixed, permeabilized, and treated with primary mouse antibodies followed by goat anti-mouse secondary antibodies conjugated to Alexa Fluor 568 (red fluorescence). The cells were subsequently counterstained with Alexa Fluor 488 conjugated to phalloidin (filamentous actin; green fluorescence) and Hoechst 33342 (DNA in the nucleus; blue fluorescence).
Histone and Peroxisome Distribution in A-10 Cell Cultures - In a double immunofluorescence experiment, an adherent culture of rat thoracic aorta myoblast cells was fixed, permeabilized, blocked with 10 percent normal goat serum, and treated with a cocktail of mouse anti-histones (pan) and rabbit anti-PMP 70 (peroxisomal membrane protein) primary antibodies, followed by goat anti-mouse and anti-rabbit secondary antibodies (IgG) conjugated to Texas Red and Alexa Fluor 488, respectively. The filamentous actin network was counterstained with Alexa Fluor 350 conjugated to phalloidin.
Concanavalin A Localization of the Endoplasmic Reticulum in Rat Thoracic Aorta Cells - A culture of adherent A-10 rat thoracic aorta cells was stained with Alexa Fluor 488 conjugated to the lectin concanavalin A, which selectively binds to alpha-mannopyranosyl and alpha-glucopyranosyl residues (primarily in the endoplasmic reticulum). Alexa Fluor 568 conjugated to phalloidin and DAPI were also used to label the culture, targeting filamentous actin and nuclear DNA, respectively.
Monolayer A-10 Cell Cultures with Oregon Green 488, MitoTracker Red CMXRos, and Hoechst 33258 - Applying a popular triple fluorophore combination, an adherent log phase culture of rat thoracic aorta cells was treated with MitoTracker Red CMXRos in growth medium for one hour, washed and fixed with paraformaldehyde (prepared in growth medium), permeabilized, and blocked with bovine serum albumen. The cells were subsequently labeled with Oregon Green 488 conjugated to phalloidin and counterstained with Hoechst 33258.
Rat Thoracic Aorta Medial Layer Myoblast Cells with Cy2, Alexa Fluor 568, and Hoechst 33258 - Focal adhesions were visualized in a log phase adherent monolayer culture of A-10 cells by immunofluorescent treatment with mouse anti-vinculin primary antibodies followed by goat anti-mouse Fab fragments conjugated to the cyanine dye, Cy2. The actin cytoskeletal network was simultaneously imaged with Alexa Fluor 568 conjugated to phalloidin, and nuclei were counterstained with Hoechst 33258.
Distribution of Vimentin and Filamentous Actin in Adherent A-10 Cell Cultures - The proximity of intermediate filaments and the cytoskeletal filamentous actin network was visualized by treating a fixed and permeabilized culture of rat thoracic aorta cells with mouse anti-vimentin primary antibodies followed by goat anti-mouse secondary antibodies (IgG) conjugated to Texas Red-X. F-actin was subsequently labeled with Alexa Fluor 350 conjugated to phalloidin, and the nuclei were counterstained with SYTOX Green.
BACK TO THE CULTURED CELLS FLUORESCENCE GALLERY
BACK TO THE FLUORESCENCE GALLERY
