Observing Mitosis with Fluorescence Microscopy
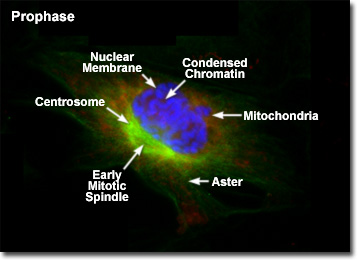
Prophase
The first stage of mitosis is known as prophase, where the nuclear chromatin starts to become organized and condenses into thick strands that eventually become chromosomes observable in the optical microscope. The nucleoli, primarily responsible for the production of ribosomal RNA, begin to disappear as the chromosomes condense. During prophase, major changes also occur in the cytoplasm, including disassembly of the cytoskeleton components based on tubulin (cytoplasmic microtubules). The tubulin monomers are dynamically redirected by the dividing cell to form the main component of the mitotic apparatus, the mitotic spindle, which is bounded by the centrosomes and begins to appear along the periphery of the nuclear membrane.
View a second, third, and fourth fluorescence image of prophase.
Presented in the digital fluorescence microscopy image above is a single rat kangaroo (PtK2) kidney cell in the early stages of prophase. The chromatin is stained with a blue fluorescent probe (DAPI), while the microtubule network (mitotic spindle) is stained green (Alexa Fluor 488) and cellular mitochondria are stained with a red dye (MitoTracker Red CMXRos). Each duplicated chromosome contains two identical sister chromatids joined together along their length, with a constricted region occurring at a specific DNA sequence known as the centromere. The chromatin in the centromere exhibits a somewhat higher degree of condensation than the rest of the chromosome. Centromeres are necessary for proper segregation of the sister chromatids during later stages of mitosis.
The mitotic spindle that begins to form in early prophase is a bipolar structure composed of microtubules and associated proteins. As the spindle grows, the centrosomes begin to translocate to opposite ends of the nucleus, apparently driven by the addition of new tubulin monomers to the existing filament network. On the molecular level, three sets of spindle microtubules can be distinguished. The polar microtubules overlap in the central region of the spindle and are responsible for pushing the poles (centrosomes) farther apart. Kinetochore microtubules attach to a specialized region within the centromeres of the sister chromatids to assist in maneuvering the chromosomes to the mitotic plate. Radiating in all directions from the centrosomes at the spindle poles are astral microtubules, which separate the poles and orient the mitotic spindle with respect to other cellular components.
In order to ensure the inheritance of a complete ensemble of critical internal components by each daughter cell, the cell division process must provide for the segregation of organelles, such as mitochondria, the Golgi apparatus, and endoplasmic reticulum during mitosis. For example, the tubular, self-contained mitochondria are unable to assemble spontaneously from their component proteins, lipids, and enzymes, and can only be duplicated by growth and fission of pre-existing organelles. In a similar manner, the large interconnected membranous complexes of the endoplasmic reticulum and Golgi apparatus require at least a portion of the original structure as a master template for duplication of the entire network. Segregation is accomplished by doubling the concentration of self-contained organelles (mitochondria) or by fragmentation, followed by vesicle formation, of the larger membrane-rich organelles (endoplasmic reticulum) during cell division. Vesicles of the endoplasmic reticulum and Golgi apparatus appear to associate to some degree with the mitotic spindle, which is probably a mechanism designed to ensure even distribution between the daughter cells.
BACK TO MITOSIS WITH FLUORESCENCE MICROSCOPY