Observing Mitosis with Fluorescence Microscopy
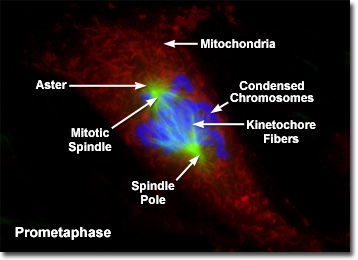
Prometaphase
Late prophase, or prometaphase, begins with the disruption of the nuclear envelope, which is broken down into small membrane vesicles that closely resemble the endoplasmic reticulum and tend to remain visible around the mitotic spindle. During this period the chromosomes continue to condense and gradually shorten and thicken until they have completely formed the units that will undergo mitosis. The nucleolus, which may still be present in some cells, also completely disappears in prometaphase.
View a second, third, and fourth fluorescence image of prometaphase.
Presented in the digital fluorescence microscopy image above is a single rat kangaroo (PtK2) kidney cell in the early stages of prometaphase. The chromatin is stained with a blue fluorescent probe (DAPI), while the microtubule network (mitotic spindle) is stained green (Alexa Fluor 488) and cellular mitochondria are stained with a red dye (MitoTracker Red CMXRos). During prometaphase, the mitotic spindle microtubules are now free to enter the nuclear region, and formation of specialized protein complexes known as kinetochores begins on each centromere. These complexes become attached to a subset of the spindle microtubules, which are then termed kinetochore microtubules. Other microtubules in the spindle (not attached to centromeres) are termed polar microtubules, and these help form and maintain the spindle structure along with astral microtubules, which remain outside the spindle.
The boundary between prophase and prometaphase is determined by the rapid onset of phosphorylation throughout the nuclear lamina triggered by activation of an enzyme termed mitosis-inducing protein kinase (abbreviated MPF). The nuclear membrane complex is disaggregated into vesicles as a result, exposing the condensed chromosomes to the expanding microtubular network of the mitotic spindle. In the optical microscope, the nuclear membrane vesicles, which are virtually indistinguishable from similar disaggregated portions of the endoplasmic reticulum, can be visualized in the region around the growing spindle.
As the kinetochore microtubules attach to their receptors on the chromatid kinetochores, the chromosomes are brought into agitated motion and move rapidly back and forth as tension is exerted by the spindle. Simultaneously, polar microtubules emanating from the centrosomes interact with each other to form an interconnecting network between the chromosomes and further establish the structure of the mitotic spindle. Kinetochores are assembled at the centromere of each chromatid to yield two kinetochores per chromosome. Eventually, at anaphase, the kinetochore microtubules will pull the sister chromatids toward opposite poles of the mitotic spindle to ensure that each daughter cell receives a complete genetic complement of chromosomes.
The complexity of the relationship between kinetochores and the mitotic spindle reflects the requirement for accurate distribution of the genetic material between dividing cells. The most common error in mitosis is the lack of separation between sister chromatids, resulting in one of the two daughter cells receiving both chromosome copies. This error, which occurs approximately once in 100,000 cell divisions, can take place if chromatids fail to attach to the correct spindle pole or if a pair of chromatids attaches to only one pole. In some cases, the two sister chromatids, although correctly assembled in the mitotic spindle apparatus, simply fail to separate at anaphase.
BACK TO MITOSIS WITH FLUORESCENCE MICROSCOPY