Fluorescence Microscopy
Brief Overview of Fluorescence Filters
The terminology applied to fluorescence filters has become a jumble as a result of various initials utilized by different manufacturers to identify their filters. In this discussion, we attempt to make some order of this confusing terminology. Basically there are three categories of filters to be sorted out: exciter filters, barrier filters and dichromatic beamsplitters (dichroic mirrors) that are usually combined to produce a filter cube similar to the one illustrated in Figure 1. Proper selection of filters is the key to successful fluorescence microscopy.
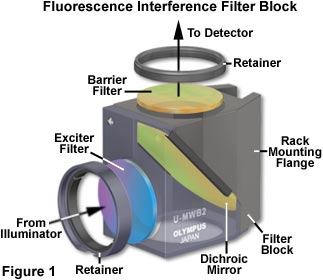
Exciter filters permit only selected wavelengths from the illuminator to pass through on the way toward the specimen. Barrier filters are filters which are designed to suppress or block (absorb) the excitation wavelengths and permit only selected emission wavelengths to pass toward the eye or other detector. Dichromatic beamsplitters (dichroic mirrors) are specialized filters which are designed to efficiently reflect excitation wavelengths and pass emission wavelengths. They are used in reflected light fluorescence illuminators and are positioned in the light path after the exciter filter but before the barrier filter. Dichromatic beamsplitters are oriented at a 45 degree angle to the light passing through the excitation filter and at a 45 degree angle to the barrier filter as illustrated in Figure 1. Filter curves (spectra) show the percentage of transmission (or the logarithm of percentage) as the vertical axis and the wavelengths as the horizontal axis.
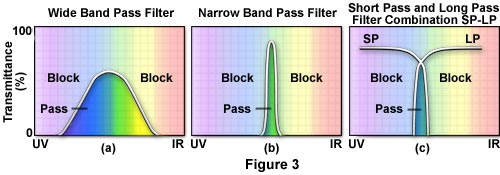
Fluorescence filters were formerly almost exclusively made of colored glass or colored gelatin sandwiched between glass. As a result of more sophisticated filter technology, interference filters have been developed that consist of dielectric coatings (of varied refractive indices and reflectivity) on glass. These filters are designed to pass or reject wavelengths of light with great selectivity and high transmission. Most of today's exciter filters are the interference type; some barrier filters are also, for special needs, the interference type. Dichromatic beamsplitters are specialized interference filters. Sometimes short pass filters (SP) and long pass (LP) filters are combined to narrow the band of wavelengths passing through such a combination. (Figure 3(c))
Exciter Filters - Abbreviations used by manufacturers to denote properties of their filters include: UG (ultraviolet glass) and BG (blue glass), which are glass exciter filters. KP (K is an abbreviation for kurz, short in German) and SP are short pass filters; and EX indicates an exciter filter.
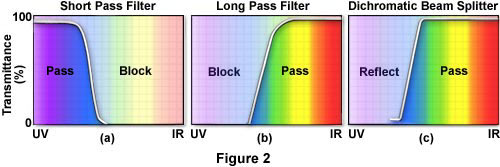
Today, most exciter filters are of the interference type. The transmission curve of a KP or SP filter shows a steep drop at the right-hand side of the curve, as illustrated in Figure 2(a). If the exciter filter is labeled with the letter B or BP, it is a band pass filter (Figure 3). A BP filter is a filter with wavelength cut-off both to the left and to the right of its curve (see Figures 3(a) and 3(b)). Numbers associated with these filters may refer to the wavelength of maximum transmission for band pass exciter filters. For SP or KP filters, the number may refer to the wavelength at 50% of the maximum transmission. For band pass filters sometimes the bandwidth, in nanometers, at the 50% level of maximum transmission is stated. Band pass filters are designed to pass only a desired band of the wavelength spectrum; many interference band pass filters pass a narrow band of the spectrum. Some manufacturers label their interference filters with the designation IF. Narrow interference band pass filters are especially helpful if the Stokes' shift is small.
| Interactive Tutorial | |||||||||||
|
|||||||||||
Barrier Filters - Acronyms or abbreviations for barrier filters include: LP or L for long pass filters, Y or GG for yellow or gelb (german) glass, R or RG for red glass, OG or O for orange glass, K for kante, a German term for edge (filter), and BA for barrier filter.
Barrier filters block (suppress) shorter wavelengths and have high transmission for longer wavelengths. When the filter type is also associated with a number, e.g. BA475, that designation refers to the wavelength (in nanometers) at 50% of its maximum transmission. Curves for barrier filters usually show a sharp edge at the left side, indicating the blocking of wavelengths to the left of that edge (see Figure 2(b)). Modern barrier filters are generally the interference type, many of which are band pass with sharp cut-offs at both the left and right sides of the transmission curve, as illustrated below in Figure 3.
Dichromatic Beamsplitters - Abbreviations used to describe and identify beamsplitters are: CBS for a chromatic beamsplitter, DM for a dichroic mirror, TK for "teiler kante", German for edge splitter, FT for "farb teiler", German for color splitter, and RKP for reflection short pass. All of these terms should be considered interchangeable.
These filters are always the interference type. The coatings are designed to have high reflectivity for shorter wavelengths and high transmission for longer wavelengths. Dichromatic beamsplitters are oriented at a 45 degree angle to the path of the excitation light entering the cube through the reflected light fluorescence illuminator. Their function is to direct the selected excitation (shorter wavelengths) light through the objective and onto the specimen. They also have the additional functions of passing longer wavelength light to the barrier filter, and reflecting any scattered excitation light back in the direction of the lamphouse.
In many of the current reflected light fluorescence illuminators, the exciter filter, the dichroic mirror, and the barrier filter are all incorporated into a single cube as illustrated in Figures 1 and 6. The illuminator, by means of a slider or rotation device, may incorporate as many as three or four cubes, thus giving the user the option to conveniently work with fluorochromes of various specifications. Alternative exciters and barriers are easily attachable for optimizing the excitation or emission of wavelengths for certain fluorochromes. The standard exciter filters and barrier filters are user-detachable so that custom-made filters can also be fitted into the microscope.
| Interactive Tutorial | |||||||||||
|
|||||||||||
Usually, the lamp housing contains an infrared or heat filter to protect the fluorescence filters from heat deterioration. Some illuminators may incorporate or accept a red suppression filter (designated BG38) to eliminate reddening of the viewfield background associated with some filter combinations. Also, the illuminator may accept a neutral density filter and/or have a opaque light shutter to reduce or temporarily block the light from reaching the specimen.
It is advisable that you inquire of the manufacturer as to what procedures they use in naming and identifying their particular filters. Samples of such nomenclature use for Olympus fluorescence microscopes are given in our data tables, but you should bear in mind that manufacturers differ in their naming rules. Microscope companies can supply the transmission curves for their exciter and barrier filters and for their dichroic mirrors. As of July, 1998, this information was not available on the websites of the major manufacturers, but we will supply links when they become available.
Cubes for Blue Excitation - To understand how a cube functions, let's take, as an example, the commonly-used cube for blue excitation. This cube (using the Olympus designation for the U-URA illuminator) is the U-MWB cube. The U-MWB cube has a band pass 450-480 exciter filter, as illustrated in Figure 4(a). This designation means that a high percentage of the excitation light falls between 450 and 480 nanometers in wavelength. The dichroic mirror in this cube is the DM500, so named because 500 nanometers is the wavelength at 50% of the maximum transmission for this mirror. The transmission curve for this mirror shows high transmission above 500 nm, a steep drop in transmission to the left of 500 nanometers, and maximum reflectivity to the left of 500 nanometers but still may have some transmission below 500 nm. The barrier filter in this cube is a BA515, that has a steep slope below 515 nanometers and thus passes little light below 515. The BA515 is a long pass filter which transmits a high percentage of wavelengths above 515 all the way up from green into the far red (Figure 4(a)).
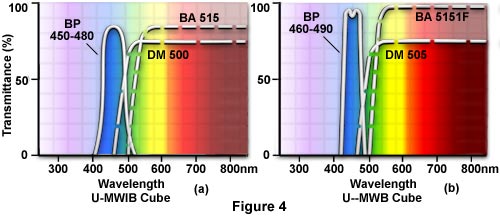
If you wish to narrow the excitation band for blue excitation, you might choose the U-MWIB cube. This cube has an interference excitation filter (very sharp slopes on either side of the excitation band) BP460, a dichroic mirror DM505 and a barrier long pass 515IF (interference barrier filter). The sharp slopes of the exciter and barrier filters do a better job in separation of excitation and emission wavelengths with minimum overlap as illustrated in Figure 4(b).
If you wished to do blue excitation but wanted to restrict the emitted wavelengths traversing the barrier filter, to green emission only, you could choose the U-MWIBBP cube. This cube has an identical exciter and dichroic mirror to the U-MWIB cube but, as its barrier filter, it has a band pass BA515-550 (NOT a long pass filter). This barrier filter passes only light in the green wavelengths between 515-550 nm and blocks longer wavelengths above 550 and blocks wavelengths shorter than 515 nm (Figure 5(a)).
| Interactive Tutorial | |||||||||||
|
|||||||||||
There are also other cubes for blue excitation, e.g. the U-MNIB and U-MNIBBP cubes that are listed with other U-URA cubes in our fluorescence cube data tables. If none of the microscope manufacturer's cubes fits your needs, you will have to go to an outside commercial manufacturer for custom-made filters and dichroic mirrors. Most microscope manufacturers now produce cubes which have removable exciter and barrier filters and a removable dichroic mirror. The function of the cube is to employ the excitation filter to tailor-make the excitation light reaching the fluorochrome; to ensure maximum reflection of the desired excitation light by the dichroic mirror; and finally to employ the barrier filter to pass the desired emission wavelengths yet block unwanted excitation light or specific unwanted emission wavelengths.
IGS Cube - In addition to the standard fluorescence cubes, manufacturers may offer a cube for immuno-gold staining. This cube, in place of a dichroic mirror, has a standard half-mirror similar to the kind used in metallurgical brightfield reflected light microscopy. In place of an exciter filter, there is positioned a long pass 420 nanometer barrier filter (to block light below 420) and a polarizing filter oriented to pass light vibrating only east-west perpendicularly to the light entering the cube. In place of a barrier filter on the cube, there is another polarizing filter (serving as an analyzer) which allows only light vibrating north-south to the light path to pass to the eye or detector. The analyzer may be placed in a not quite crossed position with respect to the polarizer. The immuno-gold (or silver) stain shows up quite clearly as it adheres to specific targets being studied.
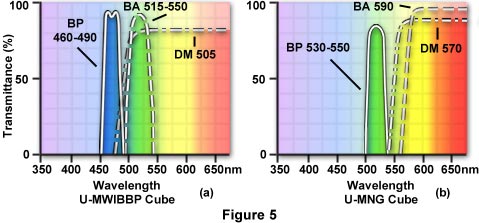
Multiple Staining - Researchers often run into crossover problems when doing multiple fluorochrome staining. For example, in the common double staining using Fluorescein isothiocyanate (FITC) and a Rhodamine conjugate, it may be that the blue excitation light exciting FITC (green emission) will also cause excitation of the Rhodamine conjugate (red emission). For this combination of stains, you might try the U-MWIBBP cube (see the data tables for filter specifications for Olympus U-URA cubes). This cube has a 460-490 nm band-pass excitation filter, which will excite FITC. The barrier filter for this particular cube is NOT a long pass filter but a band pass of 515-550nm which will restrict the emission, reaching the eye or other detector, to the green wavelengths and will block red emission from the Rhodamine.
A second cube, the U-MNG, has a band pass excitation filter 530-550 for green excitation of the Rhodamine conjugate. The barrier filter for the U-MNG cube is a long pass BA590 which will permit the red emission of the Rhodamine to reach the eye or other detector (e.g. film or video) and will block any green emission.
By alternately rotating the U-MWIBBP cube and the U-MNG cube into the light path, you should be able to separate the green emission of FITC and the red emission of Rhodamine in a double stained sample. (Figure 5) Similarly, for other combinations of multiple fluorochrome staining, the user must know the excitation-emission spectra for the fluorochromes and the transmission curves for the cubes supplied by the microscope manufacturer.

Fluorescence cube housings from different manufacturers are generally not interchangeable and are restricted for use within specific illuminators provided by the manufacturer. The cubes illustrated in Figure 6 show several designs that are currently available from Olympus and Nikon. It should be kept in mind that the individual elements (the exciter filter, barrier filter, and dichroic mirror) from the cubes of one manufacturer may sometimes be adjusted to fit into the cubes of another. This task is left for individual users to determine by trial and error.
In some instances, it may be necessary to seek custom-made filters (see data tables for sources) to secure needed excitation wavelengths and/or separation of fluorescence emission wavelengths. Several of the commercial sources now also provide custom-made filters and a dichroic mirror, installed in a single manufacturer-supplied cube, which are capable of handling double or triple fluorochrome stained specimens without crossover problems (e.g., DAPI & FITC, DAPI & FITC & TEXAS RED, pararosaniline & acriflavin, etc.)
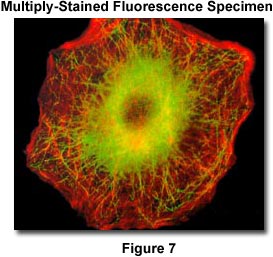
The photomicrograph illustrated in Figure 7 gives an excellent example of a double-stained specimen imaged using multiple fluorescence filters within a single cube. The sample is stained with FITC (fluorescein isothiocyanate) and Rhodamine-phalloidin to selectively highlight microtubules and actin filaments. The cubes were an Olympus WIBA and WIG combination photographed with a plan fluor 40X objective and the PM30 automatic photomicrography system.
Contributing Authors
Mortimer Abramowitz - Olympus America, Inc., Two Corporate Center Drive., Melville, New York, 11747.
Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
BACK TO FILTERS FOR FLUORESCENCE MICROSCOPY
BACK TO FLUORESCENCE MICROSCOPY
