Polarized Light Microscopy Digital Image Gallery
Bauxite
Originally discovered in Les Baux-de-Provence, France, bauxite is the world’s primary source of alumina, the oxide of metal aluminum. The rock can be found in most countries, but is most plentiful in tropical areas, such as Jamaica, and is completely absent from Canada.
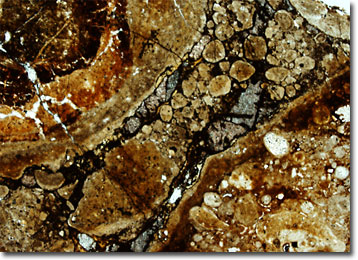
Generally formed though the weathering of a variety of rocks, in addition to hydrous aluminum oxides, bauxite may contain quartz, kaolinite, magnetite, hematite, rutile, zircon, and a number of other minerals. Due to their wide array of possible compositions, as well as to differences in geologic history, bauxites vary significantly in structure and hue. They may, for instance, be hard or soft, porous or dense, stratified or mottled, and gray, brown, yellow, red, or pink in color. Most deposits of bauxite, however, do occur near the surface of the Earth, where they can be readily obtained through open-pit mining methods.
Though first commercially mined approximately 150 years ago, aluminum was extremely expensive to derive from bauxite until Karl Joseph Bayer developed what has since come to be known as the Bayer process. Initially described in 1888, this process, which comprises several stages, involves mining ore, dissolving the alumina at an elevated temperature, adding flocculants, precipitating pure gibbsite, and excessively heating the gibbsite to yield alumina by calcination. Continued to be used in almost all modern commercial operations, the Bayer process is the primary reason that aluminum is considered a common commodity rather than a precious metal.
