Fluorescence Digital Image Gallery
Mink Uterus Endometrium Epithelial Cells (GMMe Line)
The GMMe cell line was established by the stable transfection of endometrial tissue of an adult female mink (Mustela vison) using a plasmid vector encoding the simian virus 40 (SV40) large T-antigen driven by the human beta-actin promoter. The cells were cotransfected with a second plasmid vector to confer neomycin resistance and were selected in medium containing G418.
The cuboidal GMMe cells, which exhibit epithelial characteristics, are strongly positive for cytokeratin and weakly positive for vimentin. The line is also positive for alkaline phosphatase, but negative for desmin. GMME cells have been used in co-culture with mink embryos in obligate diapause to enhance the length and frequency of embryo survival in vitro, although the mink stromal line, GMMs, was utilized in this same manner with a greater rate of success.
The endometrium is one of the three layers that comprise the uterine wall in female mammals. The mucosal epithelial lining is the innermost stratum and is functionally divided into two zones: the functionalis and the basalis. Comprised of the epithelium and glandular elements, the basalis remains in the female body on a continuous basis, but the tissue of the functionalis is built up each month and is shed during menstruation when fertilization does not occur. If an egg is successfully fertilized, however, the functionalis is not sloughed and the ovum attaches to it so that embryonic development may begin. The outer layer of the endometrium, known as the perimetrium, is composed of a mesothelium and connective tissue, and directly beneath this stratum is the myometrium, which is comprised of smooth muscle and elastic tissue.
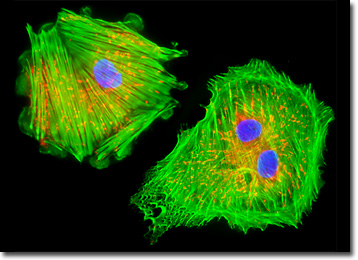
The culture of mink endometrial (GMMe) cells presented in the digital image above was labeled for mitochondria and the cytoskeletal filamentous actin network with MitoTracker Red CMXRos and Alexa Fluor 488 conjugated to phalloidin, respectively. In addition, cell nuclei were targeted with the blue fluorescent nucleic acid stain, DAPI, which preferentially stains dsDNA. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles.
Additional Fluorescence Images of Mink Uterus Endometrium (GMMe) Cells
Mink Uterus Endometrium Cells with Texas Red-X, Oregon Green 488, and Hoechst 33258 - In order to label the intermediate filaments in a log phase adherent GMMe culture, the fixed and permeabilized cells were blocked and treated with mouse anti-vimentin (porcine eye lens) primary antibodies followed by goat anti-mouse secondary antibodies (IgG) conjugated to Texas Red-X. Filamentous actin was visualized with phalloidin conjugated to Oregon Green 488, while the nuclei were stained with Hoechst 33258.
Focal Adhesion Sites in GMMe Cell Cultures - A culture of mink uterus endometrium cells was immunofluorescently labeled with primary anti-vinculin mouse monoclonal antibodies followed by goat anti-mouse secondary antibodies (IgG) conjugated to Alexa Fluor 568 (red fluorescence emission). Note the prominent staining of the cellular attachment network in the central portion and periphery of these cells. In addition, the specimen was simultaneously stained for DNA with the ultraviolet-absorbing probe Hoechst 33342, and for the cytoskeletal filamentous actin network with Alexa Fluor 488 conjugated to phalloidin.
Mink Uterus Endometrium Cells with MitoTracker Red CMXRos, Alexa Fluor 488, and DAPI - In what has become a favorite standard fluorescence staining protocol, a log phase monolayer adherent culture of GMMe cells was labeled with MitoTracker Red CMXRos before fixing, and the cells were subsequently permeabilized and stained with Alexa Fluor 488 conjugated to phalloidin, followed by DAPI, a popular DNA-binding counterstain.
BACK TO THE CULTURED CELLS FLUORESCENCE GALLERY
BACK TO THE FLUORESCENCE GALLERY
