Fluorescence Digital Image Gallery
Transformed Chicken Embryo Fibroblast Cells (UMNSAH/DF-1 Line)
UMNSAH/DF-1 is a spontaneously immortalized chicken (Gallus gallus) cell line derived from 10-day-old East Lansing strain (ELL-0) eggs. To develop the line, primary chicken embryonic fibroblasts were dissociated and grown in culture. The fibroblasts were passaged until they began to senesce.
Upon senescence, the cells were concentrated to maintain approximately 30 to 60 percent culture confluence. Foci of non-senescent cells were identified and grown for more than 30 passages. No clonal proliferation was observed in soft agar cultures, indicating that these cells were immortalized but not transformed.
The UMNSAH/DF-1 chicken embryo fibroblast line is useful as a substrate for virus propagation, recombinant protein expression, and recombinant virus production. The line is susceptible to a number of viruses, including Meleagrid herpes virus 1, fowlpox virus, reovirus, avian sarcoma leukemia virus, and Rous sarcoma virus. The cells are not, however, tumorigenic in immunosuppressed mice, but do form colonies in a semisolid medium. UMNSAH/DF-1 cells are negative for reverse transcriptase, indicating the lack of integral retrovirus genomes.
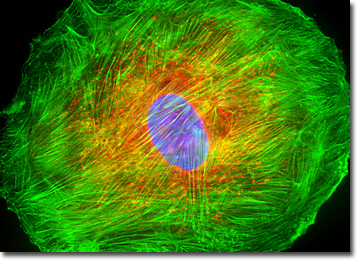
The UMNSAH/DF-1 cell culture presented in the digital image above was labeled for mitochondria and the cytoskeletal filamentous actin network with MitoTracker Red CMXRos and Alexa Fluor 488 conjugated to phalloidin, respectively. In addition, the ultraviolet-absorbing probe DAPI was utilized to target nuclear DNA. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles.
Additional Fluorescence Images of Transformed
Chicken Embryo Fibroblast Cells (UMNSAH/DF-1)
Visualizing the Microtubule and Filamentous Actin Networks in Chicken Embryo Fibroblast Cells - The microtubule network from the adherent culture of UMNSAH/DF-1 fibroblast cells presented in this section was immunofluorescently labeled with anti-tubulin mouse monoclonal primary antibodies followed by goat anti-mouse secondary antibodies (IgG) conjugated to Alexa Fluor 568 (yielding red emission). In addition, the cells were labeled for cytoskeletal F-actin with Alexa Fluor 350 (blue emission) conjugated to phalloidin, and for cell nuclei with SYTOX Green, a classic nucleic acid stain.
Histone and Peroxisome Distribution in Monolayer UMNSAH/DF-1 Cell Cultures - In a double immunofluorescence experiment, a log phase adherent monolayer culture of chicken embryo fibroblast cells was fixed, permeabilized, blocked with 10 percent normal goat serum, and treated with a cocktail of mouse anti-histones (pan) and rabbit anti-PMP 70 (peroxisomal membrane protein) primary antibodies, followed by goat anti-mouse and anti-rabbit secondary antibodies (IgG) conjugated to Texas Red and Alexa Fluor 488, respectively. The filamentous actin network was counterstained with Alexa Fluor 350 conjugated to phalloidin.
BACK TO THE CULTURED CELLS FLUORESCENCE GALLERY
BACK TO THE FLUORESCENCE GALLERY
