Loes Modderman
Sodium Chloride-Glycine Mixture
Salt has been used by mankind before recorded history as a mineral of paramount importance in regulating health, as well as in seasoning food. Sodium chloride, or common table salt, is the most familiar of the salt compounds. Also known as halite, or rock salt, this mineral forms when water evaporates from saline basins.
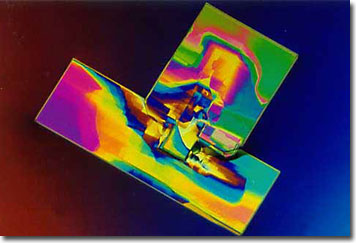
Crystallized salts are translucent, and may emit a clear hue, or a variety of other colors including white, red, yellow, orange, blue, violet, green, or gray. When crystalline formations trap bacterial debris, halites exhibit delicate pink coloring.
Table salt, the universal seasoning, is finely-grained, and comprised of high-grade sodium chloride. Many commercially sold salts, which readily absorb moisture, are supplemented with small quantities of sodium aluminosilicate, tricalcium phosphate, or magnesium silicate to ensure free-flowing granules.
Small quantities of potassium iodide are added to iodized table salts in order to prevent goiter (swelling in the thyroid gland), which results from dietary deficiencies of iodine. Salts, in addition to functioning as a seasoning for foods, are also employed as preservatives, binders, and control agents in fermentation processes. Industrial application of salts includes the manufacture of soaps and porcelain enamel, as well as other chemicals such as sodium bicarbonate (baking soda), sodium hydroxide (caustic soda), and chlorine.
Salts, which are characterized by ionic bonds, typically exhibit relatively high melting points, and a crystalline structure when occurring as a solid. When diluted in solution or in a molten state, however, the compound typically dissociates into negatively and positively charged ions that exhibit electrical conductivity, such as in the case of electrolytes. A major cation constituent in mammalian plasma, sodium is one of the crucial elements that provide for delicate electrolyte composition in body fluids. In chemistry, the term "salt" is applied to substances that are produced by a process referred to as "neutralization" wherein an acid reacts with a base.
Glycine is structurally the simplest of the 20 amino acids that combine to form proteins, possessing a single hydrogen atom in its molecular side chain. A sweet-tasting, organic compound, glycine was among the earliest amino acids to be isolated, and it is commonly found in gelatin. Although crucial for life, this amino acid is considered non-essential because it can be manufactured in the body from other amino acids, and under normal conditions, does not need to be supplemented through dietary intake. Interestingly, glycine is the only amino acid molecule that is not optically active. This compound is useful in the biosynthesis of hemoglobin, glutathione, nucleic acids, as well as serving other functions.
BACK TO LOES MODDERMAN GALLERY
