Cell Digestion and the Secretory Pathway
The primary sites of intracellular digestion are organelles known as the lysosomes, which are membrane-bounded compartments containing a variety of hydrolytic enzymes. Lysosomes maintain an internal acidic environment through the use of a hydrogen ion pump in the lysosomal membrane that drives ions from the cytoplasm into the lumenal space of the organelles. The high internal acidity is necessary for the enzymes contained in lysosomes to exhibit their optimum activity. Hence, if the integrity of a lysosomal membrane is compromised and the enzymatic contents are leaked into the cell, little damage is done due to the neutral pH of the cytoplasm. If numerous lysosomes rupture simultaneously, however, the cumulative action of their enzymes can result in autodigestion and the death of the cell.
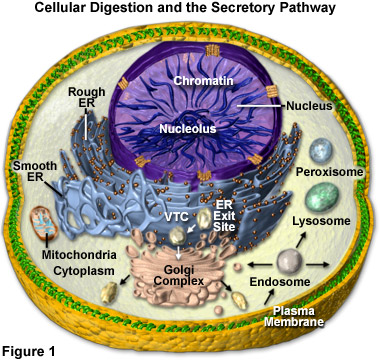
Lysosomal hydrolytic enzymes are manufactured in the rough endoplasmic reticulum (Rough ER), from whence they are transferred in a transport vesicle to the cis face of the Golgi apparatus or complex (see Figure 1). Inside of the Golgi complex, the enzymes undergo additional processing and are transformed from an inactive to an active state. As outlined in Figure 1, lysosomes may then bud from the trans face of the Golgi apparatus, though they may also form via other mechanisms (such as the gradual transformation of endosomes).
Presented in Figure 1 is a diagrammatic representation of the digestion organelles present in a typical eukaryotic cell along with the secretory pathways. The majority of the membrane and secretory proteins involved in cellular digestion are synthesized on the surface of the endoplasmic reticulum (rough ER) or are translocated to the organelle after being produced in the cytoplasm. In addition to serving as a center for protein processing and secretion, the endoplasmic reticulum forms a portion of the nuclear envelope. Newly synthesized proteins are sorted at ER exit sites (Figure 1) and enter vesicles for trafficking to intermediate compartments (VTCs), where proteins are sorted for transport to the Golgi complex or returned to the endoplasmic reticulum.
Once they arrive in the cisternal stacks of the Golgi apparatus, proteins are sorted and transported to the trans-Golgi network or sent back to earlier compartments for re-processing. Within the trans network, proteins are sorted for trafficking to either the plasma membrane, endosomes, or lysosomes (for degradation). Those proteins destined for the plasma membrane can also be recycled through the endosomes and sent back to the membrane or the Golgi, as well as sorted to late endosomes and potentially to lysosomes. Protein transfer to the mitochondria and peroxisomes in many cases probably doesn't involve processing through vesicles. The mitochondria are critical in the production of energy (via adenosine triphosphase; ATP) and lipid biosynthesis, whereas peroxisomes play a role in lipid metabolism and help reduce the intracellular concentration of free radicals.
Several different varieties of macromolecules may be digested by lysosomes and arrive at the organelles by disparate pathways. As illustrated in Figure 1, for example, many unicellular organisms and certain cells in multicellular organisms consume particles of food and other items via a process called phagocytosis. When the food is engulfed by the cell during this process, a vacuole forms around it from an invagination in the cellís plasma membrane that pinches off to completely encase the foreign material. Once released into the cell, the food vacuole is able to fuse with a lysosome. The action of the hydrolytic enzymes upon the contents of the vacuole digests them, breaking them down into monomers, such as simple sugars and amino acids, that are able to traverse the lysosomal membrane via transport proteins and enter the cytosol, where they serve as cell nutrients.
In addition to providing nutrients, the phagocytic process is important to some cell types as a mode of defense. Certain components of the vertebrate immune system, for instance, utilize phagocytosis to fight off infections. Specifically, macrophages and neutrophils, which are often referred to as scavenger cells, actively ingest bacteria and other microorganisms they encounter in the body. These intruders are then digested with the help of lysosomes.
An alternate mechanism that materials may be acquired by lysosomes for digestion is strictly intracellular. Termed autophagy, the process entails the removal of membrane-bounded organelles or other cytoplasmic components through the action of lysosomes. This digestive pathway is perhaps best illustrated in the situation where a lysosome fuses to a fractured mitochondrion. Similar to phagocytosis, the enzymes present in the lysosome disassemble the mitochondrial components into monomers and the breakdown products from autophagy are released into the cytosol.
The autophagic process is often a means of cell renewal, allowing damaged macromolecules to be recycled into products the cell can readily utilize. The liver cells, where mitochondria have an average lifetime of only about 10 days, are a good example of this function. Microscopic examination of liver cells reveals the constant presence of lysosomes containing mitochondria. Moreover, additional smooth endoplasmic reticulum produced in liver cells due to exposure to certain drugs is broken down via autophagy when the chemical substance is removed, emphasizing the fact that autophagy is a selective process.
Lysosomes are also important for their role in the programmed death of certain cells. As previously discussed, if the enzymes of a single lysosome are released into a cell, there is little change in the cytosol, but a massive enzymatic discharge by many lysosomes can be fatal to the cell. Through the coordinated release of lysosomal enzymes a number of important developmental changes occur in various multicellular organisms. As a tadpole matures into a frog, for instance, it loses its tail due to the lysosomal destruction of the cells contained in the appendage.
