Polarized Light Microscopy Digital Image Gallery
Riboflavin
Riboflavin, also known as vitamin B2, was first isolated in an unadulterated form in 1933 and was first synthesized in the laboratory approximately two years later. Since that time, synthesized riboflavin has been included in a variety of enriched foods, such as milk and cereal, and has been marketed as a vitamin supplement as well.
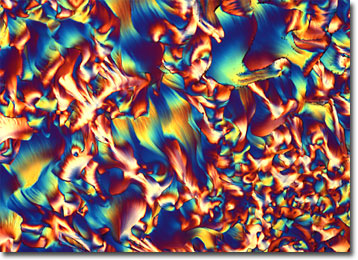
Riboflavin derives its name from the Latin word for “yellow,” an indication of the lush color of crystals formed from the pure vitamin. Biochemically, the substance, which is metabolized to form the coenzymes flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN), is involved in an array of bodily functions, such as the breakdown of fats and the syntheses of red blood cells, steroids, and glycogen. Approximately 1.2 to 1.7 milligrams of riboflavin a day is believed to be sufficient to successfully sustain such activities in the adult body. Deficiency of the vitamin is characterized by erratic symptoms that may include irritation and red cracks around the lips and mouth, swelling of the tongue, sensitivity to light, dizziness, insomnia, and hindered learning ability.
Though naturally present in a wide range of foods, such as eggs, cheese, and wheat germ, in the industrial realm riboflavin is produced via biosynthesis using yeast or other fermenting organisms. This manufactured riboflavin is then often used as a yellow coloring agent or for vitamin fortification of various foods. The vitamin is difficult, however, to integrate into many food items because it is insoluble in water. Therefore, a more costly water-soluble derivative, riboflavin-5'-phosphate, is often utilized instead. When ingested, this chemically prepared substance converts to free riboflavin.
