Polarized Light Microscopy
The First Order (Full Wave) Retardation Plate
Optical path differences ranging from a fraction of a wavelength up to several wavelengths can be readily estimated using a first order (or full wave) retardation plate. This versatile tool is known by several names, including a red plate, red-I (red-one) plate, lambda (l) plate, gypsum plate, selenite plate, sensitive violet, or simply a color tint plate, and adds a fixed optical path difference between 530 and 560 nanometers (depending upon the manufacturer) to every wavefront in the field. The first order retardation plate is a standard accessory that is frequently utilized to determine the optical sign (positive or negative) of a birefringent specimen in polarized light microscopy. In addition, the retardation plate is also useful for enhancing contrast in weakly birefringent specimens.
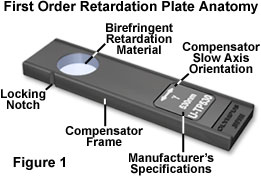
The elegantly simple anatomy of a first order retardation plate is presented in Figure 1 for a typical commercial unit. Retardation materials employed in construction of the plate vary according to the application, but usually consist of either an optical mineral thin section (such as gypsum/selenite, quartz, calcite, or mica) or a highly aligned birefringent linear organic polymer sandwiched between two isotropic optically flat glass plates. Regardless of the material used in producing retardation, the optical path difference (usually inscribed on the retardation plate frame) and optical axis orientation of the birefringent retarding material must be carefully controlled so that the plate can add a known retardation value to both the high and low refractive index azimuths. As illustrated in Figure 1, the birefringent retardation material is positioned in a rectangular frame that is inserted into the microscope optical pathway at a 45-degree angle with respect to the transmission orientations of the polarizer and analyzer. The direction of the slow (high refractive index) axis of the wavefront ellipsoid is indicated on the retardation plate frame as a double-headed arrow accompanied by the Greek symbol for "gamma" (g). In most cases, the slow axis orientation is perpendicular to the long dimension of the retardation plate frame, although this fact should be verified before attempting to use the instrument. Modern first order retardation plates are built in a frame having standardized DIN dimensions (6 x 20 millimeters) that will enable their use in a variety of microscopes.
The first order retardation plate is designed to introduce a relative retardation of exactly one wavelength (in the green or 550 nanometer region) between the ordinary and extraordinary wavefronts passing through the plate when the birefringent retardation material is illuminated by linearly polarized light at a 45-degree incident angle to the index ellipsoid. As a result, green wavelengths emerge from the retardation plate crystal still linearly polarized and having the same orientation as when they entered the retardation material (parallel to the polarizer). These wavelengths are perpendicular to the analyzer, thus are absorbed and do not pass through. The orthogonal wavefronts of all other wavelengths will experience some degree of retardation (less than a full wavelength) and will emerge from the retardation plate having varying degrees of elliptical polarization. These wavefronts are therefore able to pass a component vector through the analyzer. Subtracting the green wavelengths (blocked by the analyzer) from white light yields bright magenta-red, which results from a combination of all visible light spectral colors when the green wavelength band is missing. The magenta color observed in the microscope when a first order retardation plate is inserted into the optical train is a direct result of the events described above and is the origin for much of the common nomenclature describing this important qualitative tool.
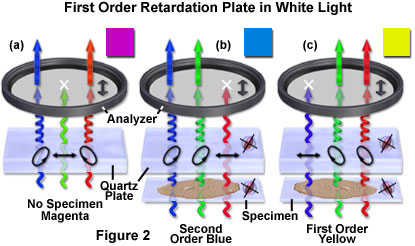
The behavior of a quartz first order retardation plate in polarized white light, symbolized by a combination of red, green, and blue wavefronts, is reviewed in Figure 2. Without a specimen in the optical pathway (Figure 2(a)), the retardation plate induces an elliptical polarization vector to the red and blue waves as they pass through, but the green light travels through the quartz crystal as a linearly polarized wavefront that is absorbed by the analyzer. As a result, only a component of the red and blue waves is able to pass through the analyzer to produce a spectrum of white light minus the green wavelengths, which is seen in the microscope as a bright magenta background.
When a birefringent specimen with a wavefront ellipsoid parallel to the retardation plate is inserted into the optical pathway (Figure 2(b)), the relative retardation of orthogonal wavefronts is increased across the viewfield so that the color (red) now exhibiting linear polarized behavior is shifted to longer wavelengths. The blue and green wavelengths are elliptically polarized and interfere at the intermediate image plane to form a hue similar to second order blue (an addition color). Rotating the specimen by 90 degrees alters the relationship between the wavefront ellipsoids (Figure 2(c)) so that they are now perpendicular. In this case, the relative retardation of the orthogonal wavefronts is decreased across the viewfield and the shorter (blue) wavelengths emerge as linearly polarized light (only to be absorbed by the analyzer). Elliptically polarized green and red wavelengths ultimately recombine to form a first order yellow (subtraction) interference color.
Inserting a first order retardation plate into the optical path of a polarized light microscope introduces a dramatic display of interference colors in thin, birefringent specimens that is not only aesthetically beautiful, but also highly useful as an indicator of several optical properties. Quantitative evaluations of relative retardation and determinations of the index ellipsoid orientation are readily achieved with a first order retardation plate. In geological and materials investigations, first order retardation plates are often employed to determine specimen thickness and to identify birefringent crystalline and polymeric materials. The tool is capable of measuring retardations with an accuracy of approximately 2 nanometers in specimens that have relatively low (one-third of a wavelength) optical path differences.
With simple uniaxial birefringent materials, the first order retardation plate can be employed to determine whether the extraordinary wavefront is slower or faster than the ordinary wavefront and thus determine the sign of birefringence. If the extraordinary wavefront is slower than the ordinary wavefront, the specimen displays positive birefringence. Conversely, a negative sign of birefringence is observed in specimens that have an ordinary wavefront that is slower than the extraordinary wavefront. First order retardation plates are ideal for use with specimens that have very low order (or only gray-level) interference colors when observed in the polarized light microscope. Before attempting an analysis of birefringence, specimens must first be oriented with the index ellipse in a diagonal position (45-degree angle) with respect to the microscope polarizer and analyzer. The two vibration (ordinary and extraordinary) azimuths will then run Northeast-Southwest and Northwest-Southeast, whereas the polarizer is oriented East-West and the analyzer North-South (as seen in the microscope viewfield).
After the specimen has been properly oriented, the colors appearing in the microscope eyepieces represent interference that is generated by the additive effects of the specimen and the first order retardation plate. If the specimen slow vibration axis is superimposed over the corresponding axis of the retardation plate, the additive retardation effects will result in higher order interference colors (termed the additive position). However, if the fast axis of the specimen is parallel to the slow axis of the retardation plate, the relative retardation will be decreased and result in lower order interference colors (the subtractive position). As an example, in a relatively thin, elongated birefringent crystal that displays only first order gray intensities under crossed polarized illumination, addition of a first order retardation plate to the optical train will result in quadrants that display blue and yellow interference colors, depending upon the orientation of the crystal. In positive crystals, yellow interference colors are observed when the crystal is oriented Southeast to Northwest, while blue colors result from rotating the crystal by 90 degrees (into a Northeast-Southwest direction). Likewise, negative crystals exhibit yellow colors when oriented Northeast-Southwest and blue colors when oriented Southeast-Northwest.
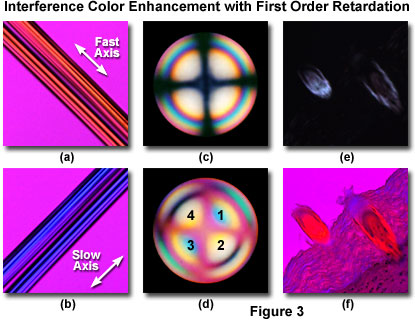
This effect is illustrated with a pair of synthetic acetate fibers in Figures 3(a) and 3(b). Without a first order retardation plate in the optical path, the fibers appear birefringent with a 140-nanometer first order gray intensity superimposed on a jet black background (not illustrated). When the long axis of the synthetic acetate is oriented Northwest-Southeast and a first order retardation plate inserted into the microscope tube, the fibers acquire a first order yellow hue on a magenta background (Figure 3(a)). Rotating the microscope stage by 90 degrees (Northeast-Southwest) alters the interference color to second order blue (Figure 3(b)). From these results, the optical sign of birefringence for the acetate fibers is judged to be positive.
The sign of birefringence can also be readily determined using a first order retardation plate coupled to observation of conoscopic interference patterns with a Bertrand lens (see Figures 3(c) and 3(d)). When a Bertrand lens is inserted into the optical train of a polarized light microscope (between the objective rear aperture and the observation tubes), the conoscopic image of a uniaxial quartz crystal appears as a series of concentric rings having increasing orders of interference fringes from the center to the periphery, which are superimposed on a darkened Maltese cross (Figure 3(c)). Inserting a first order retardation plate into the microscope nosepiece or intermediate tube divides the image into quadrants that display higher order interference colors (Figure 3(d)). If the first and third quadrants (see Figure 3(d)) of the conoscopic image produce additive interference colors (blue and higher), the crystal is positively birefringent. However, if the second and fourth quadrants add to higher interference colors, the crystal is negatively birefringent. The conoscopic image of quartz (Figure 3(d)) reveals that the optical sign of birefringence is positive in quartz having this crystalline lattice structure. Biaxial crystals can also be examined conoscopically to determine their sign of birefringence.
First order retardation plates are also efficient at increasing contrast in very weakly birefringent specimens that are difficult or impossible to detect using crossed polarized illumination alone. Many of the common birefringent biological assemblies examined in polarized light, such as cell walls, starch granules, lignin, microtubules, and actin filaments, fall into this category. An excellent example is provided by a thin section of human tongue, which displays several birefringent structures (Figures 3(e) and 3(f)) of varying intensity. In crossed polarized illumination (Figure 3(e)), weakly birefringent condensed formations at the edge of the tissue are difficult to image and striated muscle tissue supporting the structures is absent. In contrast, when a first order retardation plate is added to the optical train, the structure of all birefringent features becomes readily apparent (Figure 3(f)) and the higher order (blue and yellow) interference colors provide an indication of the optical sign.
Contributing Author
Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
BACK TO COMPENSATORS AND RETARDATION PLATES
BACK TO POLARIZED LIGHT MICROSCOPY
