 
|
Troubleshooting Photomicrography Errors
25 Most Common Errors
and
Suggested Remedies
Problem No. 1: Exposures in brightfield are shorter than 1/2 second, but the clear background of the photomicrograph appears bluish instead of white. |
Solution: The color temperature of the light source is probably too high for the film emulsion. This is one of the most common errors experienced with color photomicrography. Try using one of the Kodak 81 series Wratten filters to reduce the color temperature. If the blue background is very subtle, a low-density color compensating filter (CC10Y to CC20Y) may be sufficient to correct the problem. |
|
|
 |
 |
 |
Problem No. 2: Exposures are shorter than 1/2 second, but the clear background of the photomicrograph appears yellowish instead of white. |
Solution: The illumination color temperature is probably too low, an indication that you may be using daylight-balanced color film with a tungsten light source. Try using one of the Kodak 82 series Wratten filters to raise the color temperature. If the background tint is very subtle, a Kodak Wratten color compensating filter (10CCB or 20CCB) may be sufficient to remove the yellowish cast. If you are using daylight-balanced film, make certain a Kodak 80A (or equivalent) color-balancing filter is in place. The Olympus equivalent of this filter is the LBD and the Nikon equivalent is NCB. |
|
|
 |
 |
 |
Problem No. 3: I am using indoor/tungsten films and the exposures are below 1/2 second, but my photomicrographs are very blue overall (background and specimen). |
Solution: The color temperature of the light source is very high and not suited for the film emulsion. Make certain that you do not inadvertently have the Olympus LBD or the Kodak 80A filter in the light path. If so, remove it. |
|
|
 |
 |
 |
Problem No. 4: I am using outdoor/daylight film and the exposures are below 1/2 second, however my color micrographs are very yellow overall (both the background and the specimen). |
Solution: The light source color temperature is too low for the film emulsion. Make certain you are using an Olympus LBD, Nikon NCB or Kodak 80A conversion filter in the light path to boost the color temperature to a value of 5500 K. |
|
|
 |
 |
 |
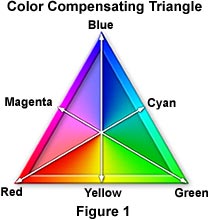
Problem No. 5: My exposures are several seconds long and resulting photomicrographs show an overall color cast (either magenta, blue, yellow, green, cyan, or red). |
Solution: Make sure you have set the reciprocity setting for the film being used and try one of the Kodak Wratten CC filters (complementary to the undesired color cast) in the light path. Consult the color compensating triangle, illustrated in Figure 1, for selection of the correct color of the compensating filter. To use the color triangle, follow the arrows from vertex to opposite side or from side to opposite vertex. Once the compensating filter color has been determined, the appropriate density must be approximated by means of test exposures. For example, a green cast is removed by the use of a CC magenta filter, with the density of the filter being dependent upon the intensity of the green cast. Likewise, color balance errors that result in yellowish highlights and shadows can be corrected with the use of a CC blue filter. |
|
|
 |
 |
 |
Problem No. 6: My image quality appears fine, but there are dark areas vignetting the corners of the photomicrographs. |
Solution: This is probably the result of failing to open the field diaphragm (in the base of the microscope at the light port) sufficiently to clear the outline of the film frame as shown in the frame finder eyepiece or focusing telescope. Make sure that, in setting up Köhler illumination, the field diaphragm has been focused on the already focused specimen, centered, and opened beyond the outline of the film frame. Do not use a photoeyepiece having a magnification factor less than 2.0, because these are meant for video imaging, not for photomicrography with film cameras. Also check the substage condenser aperture diaphragm to ensure it is opened to a value between 65 and 80 percent of the objective rear aperture diameter. |
|
|
 |
 |
 |
Problem No. 7: Although my exposures and color fidelity appear fine, the photomicrographs show an uneven intensity from one side to another. |
Solution: This probably results from having the field diaphragm and/or the aperture iris diaphragm of the condenser off center. The cause may also be that the filament of the lamp is not centered to the optical axis of the microscope. Repeat the procedure for establishing Köhler illumination, carefully monitoring the positions of the lamp filament, field diaphragm, and condenser aperture openings. Also, take out one of the eyepieces and look down the tube to see if the filament of the lamp is centered to the back lens of the objective and if the aperture iris diaphragm of the condenser appears centered, when opened and closed, to the back lens of the objective. If the microscope uses a frosted filter positioned in close proximity to the lamp, make sure it is properly inserted into the light path. Some microscopes use a pre-centered tungsten-halogen bulb, but many others have, including most older microscopes, have centering knobs on the lamphouse for centering the filament to the optical axis. |
|
|
 |
 |
 |
Problem No. 8: My exposures seem fine and the color looks right, but the photomicrographs appear blurred. |
Solution: Make sure to use a frame finder eyepiece or focusing telescope with cross hairs as a target so that accommodation of your eyes is not deceiving you to think you are in focus as you look through the microscope. If you are using an objective with a correction collar, set it for the actual thickness of the cover glass (if known). |
|
|
 |
 |
 |
Problem No. 9: Photomicrographs with the 40x and 100x objectives are excellent, but those with the 10x or 4x objectives are frequently out of focus. |
Solution: There is so much depth of field and so little depth of focus with these low magnification, low numerical aperture objectives, that you should use a focusing magnifier to insure accurate focus at the specimen level. |
|
|
 |
 |
 |
Problem No. 10: My photomicrographs generally look good overall, but I am not seeing the details of the specimen with sufficient clarity. |
Solution: You probably should be using a higher magnification, higher numerical aperture objective. Resolution is directly related to numerical aperture of the objective, and magnification helps spread the image over a greater area of the retina of the eye. Generally, in increasing total magnification, it is more desirable to use a higher magnification, higher numerical aperture objective rather than a higher magnification eyepiece. |
|
|
 |
 |
 |
Problem No. 11: My photomicrographs seem good, but in some shots, the outer areas are out of focus, while in others, the central area is out of focus. |
Solution: Your objectives are probably exhibiting curvature of field optical aberration, a common problem with older objectives that lack flat field correction. Use plan objectives, because these objectives are designed by the microscope manufacturer to deliver an image that is flat from edge to edge in the field of view. |
|
|
 |
 |
 |
Problem No. 12: My darkfield illumination photomicrographs are badly overexposed and the specimen appears washed out with little highlight detail. |
Solution: Examine the exposure adjustment on automatic camera systems to determine if the ISO is correctly set. Use the Exposure Adjust feature (if available) to reduce the exposure time, or adjust the shutter speed of SLR cameras to a lower value. An alternative method is to increase the ISO setting on both automatic and manual camera backs. When using automatic camera systems, use spot metering rather than the averaging mode for setting exposure times. Remember to place the spot meter reticle on the brightest portion of the specimen visible in the viewfield within the photomicrograph area boundaries. |
|
|
 |
 |
 |
Problem No. 13: I like to use color negative films because I can get the pictures back quickly from local photo processors, but specimen colors are coming out unlike what I see in the microscope and the background appears to have a strange color cast. |
Solution: The local processing machines have automatic color correction filters built in, but these filters are not meant for the usual stains used in microscopy. You have two choices: Use positive transparency films instead of color negative film, or arrange to show the local processor what the colors should look like and have the processor agree to hand manipulate or remove filters to get the desired result. |
|
|
 |
 |
 |
Problem No. 14: I work almost exclusively with tissue thin sections that have been stained with hematoxylin/eosin dye mixtures. The cellular nuclei appear normal, but the pink eosin stain looks pale and washed out. |
Solution: Some color films have difficulty in reproducing the pink tones of eosin stain. Try using a didymium filter in the light path to intensify the pink color of eosin. |
|
|
 |
 |
 |
Problem No. 15: Most of my work is fluorescence microscopy with daylight films, but, because of long exposure times, I have trouble getting the exposure right. |
Solution: If your camera has settings for fluorescence (Fl or SuperFl modes on the Olympus PM-30 automatic camera system; Fl on the PM-20), make sure to use them. Try spot metering the fluorescing area, and, as an extra precaution, bracket your shots over several f-stops in one-third to one-half f-stop increments. Use a fast emulsion film (e.g., T-Max 400 for black/white or Fujichrome Provia 400 for color transparencies) to reduce exposure time. Also, use high numerical aperture objectives and low magnification photo eyepieces for best results in fluorescence microscopy. |
|
|
 |
 |
 |
Problem No. 16: I use both a Polaroid 3 l/4" x 4 1/4" and a 4" x 5" film pack adapter, but my photomicrographs are all coming out black and do not appear to be exposed. What's wrong? |
Solution: These film pack adapters have a dark slide that must be pulled out of the light path; otherwise no light can reach the film for exposure. Check to make certain the dark slide is removed during exposure of the film, then reinserted after the photomicrograph has been recorded. |
|
|
 |
 |
 |
Problem No. 17: How do I find information about the reciprocity setting for specific films? |
Solution: If you are using the Olympus PM-20, the PM-30, or the U-Photo system, this information is built into the memory of these cameras and found under the film setting controls. For other less sophisticated cameras or for films of most recent production, the film manufacturer's data sheets and/or the microscope manufacturer will suggest the amount of additional exposure time needed for longer exposures. These data sheets are often found in portable document format (PDF) on the manufacturer's websites. |
|
|
 |
 |
 |
Problem No. 18: I am using a high numerical aperture objective with a correction collar, but photomicrographs appear blurred and lacking in contrast and the microscope is often difficult to focus. |
Solution: If the correction collar is properly adjusted to match coverslip thickness, check to see if the objective front lens is free of contaminating oil that may have inadvertently been acquired when the objective was swung through an oil pool after using an immersion objective. Also check to make sure the microscope slide is not positioned upside down. |
|
|
 |
 |
 |
Problem No. 19: My photomicrographs have excellent contrast and color saturation, but there are annoying dark spots on the film that are out of focus. |
Solution: Dust and debris are common problems in photomicrography. To alleviate this problem, translate the microscope slide around the stage while examining the specimen in the eyepieces. If the dark spots move with the slide, then carefully wipe both the slide and coverslip with solvent (ethanol, xylol, or lens cleaning fluid) and place it back on the microscope stage. Debris can also accumulate on the surface of lenses that are close to one of the principal conjugate planes. Check the field lens, the condenser top lens, objective front lens, eyepiece lenses, and the projection lens to ensure they are clean and dust free. Rotate the eyepieces and other optical elements in the light path to determine the source of unwanted dust and debris contamination. Also check filters that are near the field lens or underneath the substage condenser for scratches or dirt. |
|
|
 |
 |
 |
Problem No. 20: I seem to have my microscope focused properly and the eyepieces are parfocal with the projection lens, but photomicrographs still appear blurred and out of focus, especially when I use long exposure times. |
Solution: The problem may be excessive vibration in the microscope stand, or strong air currents from central heating and air conditioning units. Another source to examine is stage drift, a problem that occurs when the weight of the stage induces movement in the microscope focus rack, lowering the stage (and specimen) and causing the microscope to lose focus. Check the microscope focus tensioning mechanism (if so equipped) and adjust it to eliminate or reduce drift. Older microscopes with loose focus racks may need service by a qualified technician. |
|
|
 |
 |
 |
Problem No. 21: My photomicrographs are in good focus, but fine specimen details seem lost and the overall image contrast is low. |
Solution: This is usually an indication that the substage condenser aperture diaphragm is opened too wide. To correct this problem, remove one of the eyepieces and insert a phase telescope into the eye tube or a Bertrand lens into the light path. While observing the rear focal plane of the objective, adjust the diaphragm until its diameter lies between 65 and 80 percent of the objective aperture. When using a reflected light microscope, adjust both the field diaphragm and the aperture diaphragm to improve contrast. |
|
|
 |
 |
 |
Problem No. 22: Some of my photomicrographs have sharply focused, dark corners with straight edges, but overall image quality is good. |
Solution: The problem is probably due to interference by the field diaphragm. Check to see if the diaphragm is opened sufficiently to remain out of the viewfield, yet closed to the point of reducing undesirable glare. If adjustment of the field diaphragm does not cure the problem, then it may be vignetting caused by the projection lens. Examine the projection lens to ensure it is properly seated and is of sufficient magnification to completely fill the diagonal of the film frame in order to avoid vignetting. Microscope manufacturers usually offer a range of projection lenses in differing magnifications from 0.5x to 7.5x, with incremental sizes in between. |
|
|
 |
 |
 |
Problem No. 23: Specimens in my microscope appear fine and the camera system seems to work properly, but micrographs are so dark that I am not sure the film has even been exposed. |
Solution: When the film is very dark or totally black, there is a possibility that it has not been exposed. This can be indicative of a serious problem with the film advance mechanism on automatic cameras or a malfunction of the camera shutter system. If the entire roll is unexposed, yet the camera appeared to be functioning properly during photomicrography, check to make certain the camera is receiving light from the microscope. Also examine the take-up spool to make sure it is working properly and the film is correctly attached. Many automatic exposure systems will not allow film to be exposed when insufficient light is being received, but older manual systems do not have this safety feature. In film rolls where only a single or couple of frames are unexposed, check the camera to make sure film is advancing properly. |
|
|
 |
 |
 |
Problem No. 24: Photomicrographs appear very dark, and although there is some detail in the highlight regions, these areas are off-color because of unwanted yellow and gray casts and shadows are completely black with indiscernible features. |
Solution: The film is probably not receiving enough light to properly expose the emulsion. Color transparency film is not very forgiving of exposure errors and will only produce acceptable results within a half to one f-stop of the correct exposure. Digital images and color negative films provide a greater exposure latitude, but these media are also subject to underexposure errors. If the average specimen brightness is not uniform over the entire viewfield, microscope camera systems with automatic exposure features may cause underexposure of specimens with very bright backgrounds. Use a spot meter to alleviate this problem, or if the electronic camera meter is constrained to average too much of the viewfield, lower the film speed setting to increase exposure times. It is always a good idea to calibrate new specimens and film by bracketing exposures in one-third to one-half f-stop increments. Digital cameras are also prone to underexposure errors when the camera metering system is confused by uneven specimen illumination. |
|
|
 |
 |
 |
Problem No. 25: My color transparencies appear very thin and washed out. In addition, highlight regions suffer from a significant loss of image detail. |
Solution: This error occurs because the film has received too much light, and is usually caused by shutter speeds that are too slow or excess illumination intensity. Tungsten-halogen lamps (12-volt and 50- or 100-watt) should be adjusted to a setting between 8.5 and 9.0 volts for optimum photomicrography color temperature and illumination. If the entire roll is overexposed, the fault is probably in the exposure settings on the camera system. Check the camera ISO indicator to ensure it matches the film speed and take note of the shutter speed on manual cameras. Automatic camera systems can overexpose film if their lowest setting has a shutter speed too long for the amount of illumination. Reduce illumination intensity with neutral density filters, and perform test brackets to dial in exposure settings when using new specimens or film. |
|
|
 |
 |
 |
Contributing Authors
Mortimer Abramowitz - Olympus America, Inc., Two Corporate Center Drive., Melville, New York, 11747.
Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
BACK TO PHOTOMICROGRAPHY ERRORS
BACK TO PHOTOMICROGRAPHY
Questions or comments? Send us an email.
© 1998-2025 by
Michael W. Davidson and The Florida State University.
All Rights Reserved. No images, graphics, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
Last modification: Friday, Nov 13, 2015 at 01:19 PM
Access Count Since July 5, 2000: 33734
For more information on microscope manufacturers,
use the buttons below to navigate to their websites:




|

