Light: Particle or a Wave?
The exact nature of visible light is a mystery that has puzzled man for centuries. Greek scientists from the ancient Pythagorean discipline postulated that every visible object emits a steady stream of particles, while Aristotle concluded that light travels in a manner similar to waves in the ocean. Even though these ideas have undergone numerous modifications and a significant degree of evolution over the past 20 centuries, the essence of the dispute established by the Greek philosophers remains to this day.
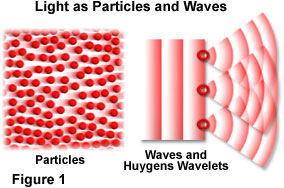
One point of view envisions light as wave-like in nature, producing energy that traverses through space in a manner similar to the ripples spreading across the surface of a still pond after being disturbed by a dropped rock. The opposing view holds that light is composed of a steady stream of particles, much like tiny droplets of water sprayed from a garden hose nozzle. During the past few centuries, the consensus of opinion has wavered with one view prevailing for a period of time, only to be overturned by evidence for the other. Only during the first decades of the twentieth century was enough compelling evidence collected to provide a comprehensive answer, and to everyone's surprise, both theories turned out to be correct, at least in part.
In the early eighteenth century, the argument about the nature of light had turned the scientific community into divided camps that fought vigorously over the validity of their favorite theories. One group of scientists, who subscribed to the wave theory, centered their arguments on the discoveries of Dutchman Christiaan Huygens. The opposing camp cited Sir Isaac Newton's prism experiments as proof that light traveled as a shower of particles, each proceeding in a straight line until it was refracted, absorbed, reflected, diffracted or disturbed in some other manner. Although Newton, himself, appeared to have some doubt about his corpuscular theory on the nature of light, his prestige in the scientific community held so much weight that his advocates ignored all other evidence during their ferocious battles.
Huygens' theory of light refraction, based on the concept of the wave-like nature of light, held that the velocity of light in any substance was inversely proportion to its refractive index. In other words, Huygens postulated that the more light was "bent" or refracted by a substance, the slower it would move while traversing across that substance. His followers concluded that if light were composed of a stream of particles, then the opposite effect would occur because light entering a denser medium would be attracted by molecules in the medium and experience an increase, rather than a decrease, in speed. Although the perfect solution to this argument would be to measure the speed of light in different substances, air and glass for example, the devices of the period were not up to the task. Light appeared to move at the same speed regardless of the material through which it passed. Over 150 years passed before the speed of light could be measured with a high enough accuracy to prove that the Huygens theory was correct.
Despite the highly regarded reputation of Sir Isaac Newton, a number of prominent scientists in the early 1700s did not agree with his corpuscular theory. Some argued that if light consisted of particles, then when two beams are crossed, some of the particles would collide with each other to produce a deviation in the light beams. Obviously, this is not the case, so they concluded that light must not be composed of individual particles.
| Interactive Tutorial | |||||||||||
|
|||||||||||
Huygens, for all his intuition, had suggested in his 1690 treatise Traité de la Lumière that light waves traveled through space mediated by the ether, a mystical weightless substance, which exists as an invisible entity throughout air and space. The search for ether consumed a significant amount of resources during the nineteenth century before finally being laid to rest. The ether theory lasted at least until the late 1800s, as evidenced by Charles Wheatstone's proposed model demonstrating that ether carried light waves by vibrating at an angle perpendicular to the direction of light propagation, and James Clerk Maxwell's detailed models describing the construction of the invisible substance. Huygens believed that ether vibrated in the same direction as light, and formed a wave itself as it carried the light waves. In a later volume, Huygens' Principle, he ingeniously described how each point on a wave could produce its own wavelets, which then add together to form a wavefront. Huygens employed this idea to produce a detailed theory for the refraction phenomenon, and also to explain why light rays do not crash into each other when they cross paths.
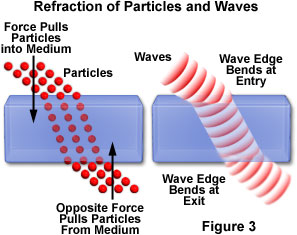
When a beam of light travels between two media having different refractive indices, the beam undergoes refraction, and changes direction when it passes from the first medium into the second. To determine whether the light beam is composed of waves or particles, a model for each can be devised to explain the phenomenon (Figure 3). According to Huygens' wave theory, a small portion of each angled wavefront should impact the second medium before the rest of the front reaches the interface. This portion will start to move through the second medium while the rest of the wave is still traveling in the first medium, but will move more slowly due to the higher refractive index of the second medium. Because the wavefront is now traveling at two different speeds, it will bend into the second medium, thus changing the angle of propagation. In contrast, particle theory has a rather difficult time explaining why particles of light should change direction when they pass from one medium into another. Proponents of the theory suggest that a special force, directed perpendicular to the interface, acts to change the speed of the particles as they enter the second medium. The exact nature of this force was left to speculation, and no evidence has ever been collected to prove the theory.
Another excellent comparison of the two theories involves the differences that occur when light is reflected from a smooth, specular surface, such as a mirror. Wave theory speculates that a light source emits light waves that spread in all directions. Upon impacting a mirror, the waves are reflected according to the arrival angles, but with each wave turned back to front to produce a reversed image (Figure 4). The shape of arriving waves is strongly dependent upon how far the light source is from the mirror. Light originating from a close source still maintains a spherical, highly curved wavefront, while light emitted from a distance source will spread more and impact the mirror with wavefronts that are almost planar.
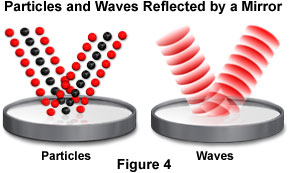
The case for a particle nature for light is far stronger with regards to the reflection phenomenon than it is for refraction. Light emitted by a source, whether near or far, arrives at the mirror surface as a stream of particles, which bounce away or are reflected from the smooth surface. Because the particles are very tiny, a huge number are involved in a propagating light beam, where they travel side by side very close together. Upon impacting the mirror, the particles bounce from different points, so their order in the light beam is reversed upon reflection to produce a reversed image, as demonstrated in Figure 4. Both the particle and wave theories adequately explain reflection from a smooth surface. However, the particle theory also suggests that if the surface is very rough, the particles bounce away at a variety of angles, scattering the light. This theory fits very closely to experimental observation.
| Interactive Tutorial | |||||||||||
|
|||||||||||
Particles and waves should also behave differently when they encounter the edge of an object and form a shadow (Figure 5). Newton was quick to point out in his 1704 book Opticks, that "Light is never known to follow crooked passages nor to bend into the shadow". This concept is consistent with the particle theory, which proposes that light particles must always travel in straight lines. If the particles encounter the edge of a barrier, then they will cast a shadow because the particles not blocked by the barrier continue on in a straight line and cannot spread out behind the edge. On a macroscopic scale, this observation is almost correct, but it does not agree with the results obtained from light diffraction experiments on a much smaller scale.
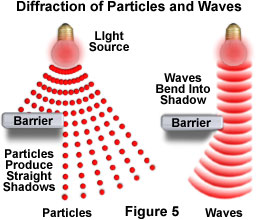
When light is passed through a narrow slit, the beam spreads and becomes wider than expected. This fundamentally important observation lends a significant amount of credibility to the wave theory of light. Like waves in water, light waves encountering the edge of an object appear to bend around the edge and into its geometric shadow, which is a region that is not directly illuminated by the light beam. This behavior is analogous to water waves that wrap around the end of a raft, instead of reflecting away.
Almost a hundred years after Newton and Huygens proposed their theories, an English physicist named Thomas Young performed an experiment that strongly supported the wave-like nature of light. Because he believed that light was composed of waves, Young reasoned that some type of interaction would occur when two light waves met. In order to test this hypothesis, he used a screen containing a single, narrow slit to produce a coherent light beam (containing waves that propagate in phase) from ordinary sunlight. When the sun's rays encounter the slit, they spread out or diffract to produce a single wavefront. If this front is allowed to illuminate a second screen having two closely spaced slits, two additional sources of coherent light, perfectly in step with each other are produced (see Figure 6). Light from each slit traveling to a single point halfway between the two slits should arrive perfectly in step. The resulting waves should reinforce each other to produce a much larger wave. However, if a point on either side of the central point is considered, then light from one slit must travel much farther to reach a second point on the opposite side of the central point. Light from the slit closer to this second point would arrive before light from the distant slit, so the two waves would be out of step with each other, and might cancel each other to produce darkness.
| Interactive Tutorial | |||||||||||
|
|||||||||||
As he suspected, Young discovered that when the light waves from the second set of slits are spread (or diffracted), they meet each other and overlap. In some cases, the overlap combines the two waves exactly in step. However, in other cases, the light waves are combined either slightly or completely out of step with each other. Young found that when the waves met in step, they added together by a process that has come to be termed constructive interference. Waves that meet out of step will cancel each other out, a phenomenon known as destructive interference. In between these two extremes, various degrees of constructive and destructive interference occur to produce waves having a wide spectrum of amplitudes. Young was able to observe the effects of interference on a screen placed at a set distance behind the two slits. After being diffracted, the light that is recombined by interference produces a series of bright and dark fringes along the length of the screen.
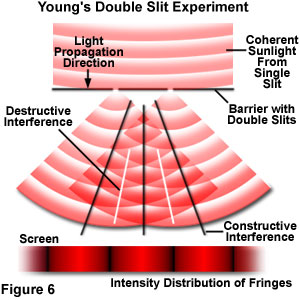
Although seemingly important, Young's conclusions were not widely accepted at the time, primarily because of the overwhelming belief in the particle theory. In addition to his observations on light interference, Young postulated that light of different colors was composed of waves having different lengths, a fundamental concept that is widely accepted today. In contrast, the particle theory advocates envisioned that various colors were derived from particles having either different masses or traveling at different speeds.
The interference effect is not restricted to light. Waves produced on the surface of a pool or pond will spread in all directions and undergo an identical behavior. Where two waves meet in step, they will add together to make a larger wave by constructive interference. Colliding waves that are out of step will cancel each other via destructive interference and produce a level surface on the water.
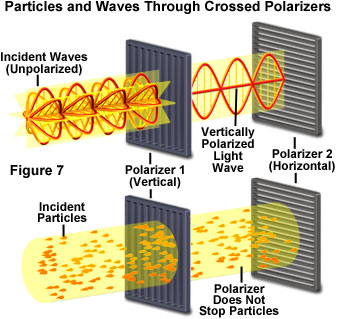
Even more evidence for a wave-like nature of light was uncovered when the behavior of a light beam between crossed polarizers was carefully examined (Figure 7). Polarizing filters have a unique molecular structure that allows only light having a single orientation to pass through. In other words, a polarizer can be considered a specialized type of molecular Venetian blind having tiny rows of slats that are oriented in a single direction within the polarizing material. If a beam of light is allowed to impact a polarizer, only light rays oriented parallel to the polarizing direction are able to pass through the polarizer. If a second polarizer is positioned behind the first and oriented in the same direction, then light passing through the first polarizer will also pass through the second.
| Interactive Tutorial | |||||||||||
|
|||||||||||
However, if the second polarizer is rotated at a small angle, the amount of light passing through will be decreased. When the second polarizer is rotated so the orientation is perpendicular to that of the first polarizer, then none of the light passing through the first polarizer will pass through the second. This effect is easily explained with the wave theory, but no manipulation of the particle theory can explain how light is blocked by the second polarizer. In fact, the particle theory is also not adequate to explain interference and diffraction, effects that would be later found to be manifestations of the same phenomenon.
The effects observed with polarized light were critical to the development of the concept that light consists of transverse waves having components that are perpendicular to the direction of propagation. Each of the transverse components must have a specific orientation direction that enables it to either pass through or to be blocked by a polarizer. Only those waves with a transverse component parallel to the polarizing filter will pass through, and all others will be blocked.
By the middle of the 1800s, scientists were becoming increasingly convinced of the wave-like character of light, but there remained one overbearing problem. Exactly what is light? A breakthrough was made when it was discovered by English physicist James Clerk Maxwell that all forms of electromagnetic radiation represent a continuous spectrum, and travel through a vacuum at the same speed: 186,000 miles per second. Maxwell's discovery effectively nailed the coffin of the particle theory and, by the dawn of the twentieth century, it seemed that the basic questions of light and optical theory had finally been answered.
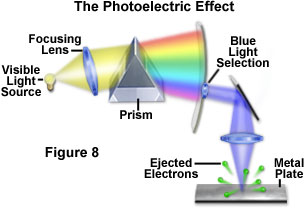
A major blow to the wave theory occurred behind the scenes in the late 1880s when scientists first discovered that, under certain conditions, light could dislodge electrons from the atoms of several metals (Figure 8). Although at first only a curious and unexplainable phenomenon, it was quickly discovered that ultraviolet light could relieve atoms of electrons in a wide variety of metals to produce a positive electrical charge. German physicist Philipp Lenard became interested in these observations, which he termed the photoelectric effect. Lenard used a prism to split white light into its component colors, and then selectively focused each color onto a metal plate to expel electrons.
What Lenard discovered confused and amazed him. For a specific wavelength of light (blue, for example), the electrons produced a constant potential, or a fixed amount of energy. Decreasing or increasing the amount of light produced a corresponding increase or decrease in the number of electrons liberated, but each still maintained the same energy. In other words, electrons escaping their atomic bonds had energies that were dependent on the wavelength of light, not the intensity. This is contrary to what would be expected from the wave theory. Lenard also discovered a link between wavelength and energy: shorter wavelengths produced electrons having greater amounts of energy.
The foundation for a connection between light and atoms was cast in the early 1800s when William Hyde Wollaston discovered that the sun's spectrum was not a continuous band of light, but contained hundreds of missing wavelengths. Over 500 narrow lines corresponding to missing wavelengths were mapped by German physicist Joseph von Fraunhofer, who assigned letters to the largest gaps. Later, it was discovered that the gaps were produced from absorption of specific wavelengths by atoms in the sun's outer layer. These observations were some of the first links between atoms and light, although the fundamental impact was not understood at the time.
In 1905, Albert Einstein postulated that light might actually have some particle characteristics, regardless of the overwhelming evidence for a wave-like nature. In developing his quantum theory, Einstein suggested mathematically that electrons attached to atoms in a metal can absorb a specific quantity of light (first termed a quantum, but later changed to a photon) and thus have the energy to escape. He also speculated that if the energy of a photon were inversely proportional to the wavelength, then shorter wavelengths would produce electrons having higher energies, a hypothesis borne in fact from the results of Lenard's research.
Einstein's theory was solidified in the 1920s by the experiments of American physicist Arthur H. Compton, who demonstrated that photons had momentum, a necessary requisite to support the theory that matter and energy are interchangeable. About the same time, French scientist Louis-Victor de Broglie proposed that all matter and radiation have properties that resemble both a particle and a wave. De Broglie, following Max Planck's lead, extrapolated Einstein's famous formula relating mass and energy to include Planck's constant:
where E is the energy of a particle, m the mass, c is the speed of light, h is Planck's constant, and n is the frequency. De Broglie's work, which relates the frequency of a wave to the energy and mass of a particle, was fundamental in the development of a new field that would ultimately be utilized to explain both the wave-like and particle-like nature of light. Quantum mechanics was born from the research of Einstein, Planck, de Broglie, Neils Bohr, Erwin Schrödinger, and others who attempted to explain how electromagnetic radiation can display what has now been termed duality, or both particle-like and wave-like behavior. At times light behaves as a particle, and at other times as a wave. This complementary, or dual, role for the behavior of light can be employed to describe all of the known characteristics that have been observed experimentally, ranging from refraction, reflection, interference, and diffraction, to the results with polarized light and the photoelectric effect. Combined, the properties of light work together and allow us to observe the beauty of the universe.
Contributing Authors
Kenneth R. Spring - Scientific Consultant, Lusby, Maryland, 20657.
Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
BACK TO LIGHT: PARTICLE OR A WAVE?
