Spike (M.I.) Walker
Carbon Composite
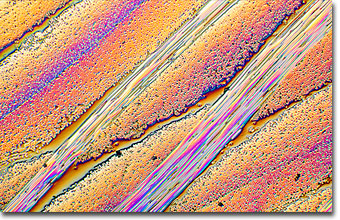
English photomicrographer Spike (M.I.) Walker has been a consistent winner of the Nikon Small World competition for many years and has published many articles and a book about microscopy. Featured below is a reflected differential interference contrast (DIC) photomicrograph of a carbon composite.
|
The carbon/carbon composite photomicrograph presented reveals numerous bundles of carbon fibers embedded in graphite. A photograph of this particular specimen was entered in a Polaroid photomicrographic competition and Spike Walker was so impressed with the material that he explored microscopy on the original sample. The objective was an epi plan achromat 4x/0.10 NA (polarizing) coupled to a vertical illuminator (Zeiss epicondenser IIC) operating in differential interference contrast (DIC). The microscope was a Zeiss Ultraphot III with an automatic 35-millimeter photohead. The film was Fujichrome Velvia. (2.5x) |
Carbon is a nonmetallic chemical element, one of the five chemical elements that make up Group IVa of the periodic table. Elemental carbon is actually a minor component of the Earth's crust, but it is one of the more abundant elements in the universe. Only hydrogen, helium, oxygen, neon, and nitrogen are more abundant.
This element is essential to life, its atoms forming the substrate for all the major molecules that life on Earth possible, including sugars, proteins, fats, and deoxyribonucleic acid (DNA), the molecular structure that carries the genetic code of living organisms.
Carbon is allotropic, that is, it exists in more than one form: amorphous carbon such as charcoal, coal, and coke; crystals such as graphite and diamonds; and fullerenes, a soccer ball-like arrangement of carbon atoms.
Each of the amorphous forms of carbon has its own qualities and, consequently, its own applications range from dyes and inks to fuels and filtering agents. Although both diamond and graphite are crystalline in structure, they display dramatically different physical properties because of the way their atoms are structured.
Pure diamond is the hardest naturally occurring substance known. While it is a poor conductor of electricity, it is the best natural conductor of heat known, three or four times better than copper or silver. Diamonds are valued as jewels and, because of their hardness, as abrasives for cutting, grinding, and drilling.
Graphite is a soft, slippery solid that is a good conductor of both heat and electricity. It is used as a lubricant, in paint, and, mixed with clay, as the "lead" of pencils. Because it conducts electricity without melting, graphite is used for electrodes in electric furnaces and dry cells as well as for making crucibles in which metals are melted.
Fullerenes are the third form of pure carbon known to exist, in addition to diamond and graphite. Buckminsterfullerene was the first fullerene discovered, in 1985 by Richard E. Smalley and Robert F. Curl, Jr., of the United States and Sir Harold W. Kroto of the United Kingdom. The unique structure and properties of buckminsterfullerene suggests potential uses for fullerenes as superconductors, lubricants, industrial catalysts, and drug-delivery systems for medical applications.
