Karl E. Deckart
Soap Bubble Gallery: Image Nineteen
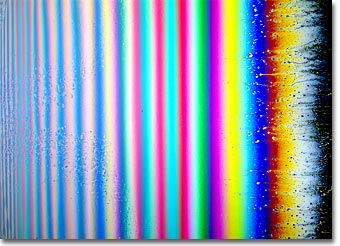
German photographer and artist Karl E. Deckart is known for his thorough, precise, and beautiful work both in photography through the microscope and with macro camera systems. This gallery of interference photographs made with soap films is a testament to both Deckart's skill as a photographer and his understanding of the physical phenomena that surround our everyday lives. Presented below is soap bubble image number nineteen in small format. Click on the image to download a larger version.
|
Macrophotography of thin soap films freely suspended on a 4 x 4-inch wire frame was conducted with a Linhof large-format bellows camera system utilizing 4 x 5-inch sheet film and imaged using an apo-macro Nikon large format Nikkor-AM ED 210 mm f-5.6 lens. To prepare the soap film, equal parts of water, glycerin, and dishwasher detergent are thoroughly mixed in a container until a solution containing evenly sized micelles is achieved. A freestanding film is formed by dipping the wire frame into the solution and withdrawing carefully to maintain an even film thickness and avoid disruption of material flow across the frame rails. After suspension, the film was illuminated by a reflected light source positioned a few degrees from the camera system. The light was passed through a diffusion screen to avoid bright spots and provide an even illumination across the field. No polarizers were employed in photomacrography of soap thin films. Image ©1999 by Karl E. Deckart. All rights reserved. |
Soap is commonly found in shampoo, bar soap, dishwashing liquid and floor cleaner, among a variety of other products. Probably discovered by mistake, the cleaning action achieved by a combination of water, oil, and alkali-containing substances can easily dissolve greasy dirt from clothing and skin where water alone cannot. For centuries, soap has been made by boiling animal fats or vegetable oil with a strong alkali such as sodium hydroxide derived from the ashes of wood or other plants. Alkali is an Arabic word meaning "ashes of the plant," which is today produced by electrolysis. In general soap making, fatty acids are separated from glycerol during boiling so that the oils can react with alkali and produce cleaning agents. Brine is later added to the solution to dissolve glycerin, allowing the soap content to rise to the surface as a curd. Additional chemicals are added to kill bacteria and add color or scent. Specialized dyes called "brighteners" are often added to detergents to make the colors in textiles appear vivid.
BACK TO THE SOAP BUBBLE GALLERY
