Karl E. Deckart
Soap Bubble Gallery: Image Twelve
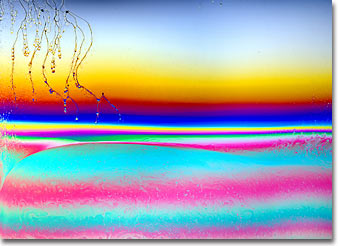
German photographer and artist Karl E. Deckart is known for his thorough, precise, and beautiful work both in photography through the microscope and with macro camera systems. This gallery of interference photographs made with soap films is a testament to both Deckart's skill as a photographer and his understanding of the physical phenomena that surround our everyday lives. Presented below is soap bubble image number twelve in small format. Click on the image to download a larger version.
|
Macrophotography of thin soap films freely suspended on a 4 x 4-inch wire frame was conducted with a Linhof large-format bellows camera system utilizing 4 x 5-inch sheet film and imaged using an apo-macro Nikon large format Nikkor-AM ED 210 mm f-5.6 lens. To prepare the soap film, equal parts of water, glycerin, and dishwasher detergent are thoroughly mixed in a container until a solution containing evenly sized micelles is achieved. A freestanding film is formed by dipping the wire frame into the solution and withdrawing carefully to maintain an even film thickness and avoid disruption of material flow across the frame rails. After suspension, the film was illuminated by a reflected light source positioned a few degrees from the camera system. The light was passed through a diffusion screen to avoid bright spots and provide an even illumination across the field. No polarizers were employed in photomacrography of soap thin films. Image ©1999 by Karl E. Deckart. All rights reserved. |
Soap cleans by loosening grease and allowing a rush of water to wash dirt away. Most soaps and detergents are comprised of long-chained molecules containing hydrogen, oxygen and carbon. One end of the soap molecule is termed hydrophilic and is comprised of atoms that cling to water molecules. The other hydrophobic end repels water and readily attaches to grease. The long molecules in soap work by surrounding and penetrating grease particles with their hydrophobic tails. In high concentrations, soap molecules aggregate to form micelles that surround dirt particles but are able to stay dispersed in water because of the hydrophilic portions, which encapsulate the micelles and form hydrogen bonds with water molecules. Synthetically developed detergents go a step further by incorporating complex chemicals that better penetrate dirt by reducing surface tension to make water "wetter." Many washing agents also contain enzymes that break down stains comprised of proteins.
BACK TO THE SOAP BUBBLE GALLERY
