
Zinc-Carbon Batteries
The zinc-carbon battery or dry cell is the technological foundation of today's growing battery industry.
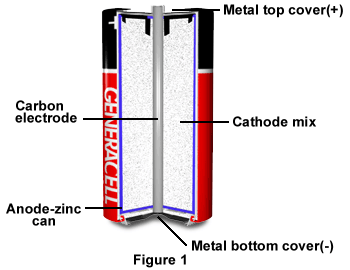
The components of the zinc-carbon battery are housed within a solid zinc can, which also serves as the battery's anode (Figure 1). The cathode mix is usually a moist substance of manganese dioxide powder, special carbon black, an electrolyte, and solution blended together. A carbon electrode rod runs down the middle, and the positive top cover and negative bottom cover are both metal. Zinc-carbon batteries usually provide 1.4 to 1.7 volts of D.C. electric power that gradually declines to .9 volts during use. The cells aren’t affected by the many impurities their ingredients contain, and remain inexpensive whether used on heavy or light electrical loads. Despite their low cost, the cells have excellent shelf life, and have very low leakage when unused for long periods of time.
History of the Zinc-Carbon CellThe zinc-carbon cell, or dry cell, is the forefather of today’s cells, and is often called the Leclanche cell after its inventor, Georges Leclanche. The original Leclanche cell utilized only one liquid material, an ammonium chloride solution that replaced the acid electrolyte used in earlier cells. A manganese dioxide and carbon dry mix replaced the depolarizing solution of most previous cells, and a carbon bar, whose function was both a current collector and positive electrode, went down the middle. At its invention, it was restricted to laboratories due to its liquid content.
The first dry cell, also a zinc-carbon cell, appeared between 1886 and 1888, and was developed by Karl Gassner. At first, the electrolyte was composed of a paste made up of zinc oxide, sal ammoniac, and water, and the zinc negative electrode was also the container for the cell’s contents. The carbon rod went down the center of the battery, and served as its positive electrode.
ChemistryThe zinc-carbon cell has a zinc anode, a manganese dioxide cathode, and an electrolyte of ammonium chloride or zinc chloride, which is dissolved in water. For each unit of electrical energy a galvanic cell creates, an equivalent amount of electrode material salts must move or be altered to provide energy. Ammonium chloride and zinc chloride in an aqueous solution combine to form a moist mixture: the cathode contains solid ammonium chloride, which acts as a fuel reserve for the cell during intermittent operation, and materials such as gum karaya and ion exchange resins may be added to the cathode in order to increase the discharge efficiency. In addition, zinc carbon cells contain separators up to 3.5 mm thick that are made of cereal paste and electrolyte solution, and serve as an electrolyte reservoir as well as a membrane between the electrodes.
Types of Zinc-Carbon CellsZinc-carbon dry cells are sold in two main classes: cylindrical cells and flat cells. The cylindrical cells come either singly or with two more in a battery, while flat cells are usually sold from four to three hundred or more cells in a stack or set of stacks.
Construction DetailsZinc-carbon batteries have a variety of electrode and packaging materials-- each material must be of high quality, or the performance of the cell or its appearance will be degraded to some extent. Most dry cells combine zinc with mercury (less than 1 part per million in modern cells) to significantly improve its resistance to corrosion over times. The zinc may contain about 0.05% cadmium, as the cadmium refines the grain and makes the alloy harder and also more corrosion resistant, and may also contain 0.25% lead. Note, cadmium and mercury have been banned from most consumer batteries of this type manufactured in the United States since 1990 because of environmental concerns associated with their disposal.
The manganese dioxide cathode material is another important component, and must be very pure. Usually, this compound comes from mines in Mexico, Gabon, China, and Brazil, where impurities like nickel, copper, arsenic, and cobalt are in small quantities or insoluble. The manganese dioxide is always mixed with graphite or acetylene (carbon) black to provide better electrolyte conductivity and absorption. Usually, only a small amount of graphite is used, with the majority of the carbon as acetylene black, because it is a stable form of finely divided carbon and is highly conductive.