
Zinc-Air Batteries
Zinc-air batteries employ oxygen from the air to use in their cathode, and use an anode primarily composed of zinc and an alkaline electrolyte.
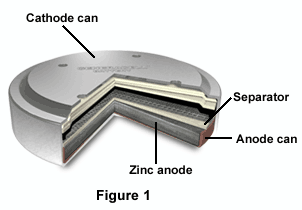
Figure 1 shows a cut-out of a zinc-air button cell. The anode is usually comprised of a granulated powder mixed with electrolyte, and often employs a gelling agent to cause contact between the electrolyte and the zinc granules. The metal can halves function as the positive and negative terminals, and a plastic gasket is used as an insulator between the two halves. The small cathode allows twice as much zinc anode to be used by the battery, therefore increasing its output potential. The battery's electrical capacity is determined by anode capacity, and its energy density is at least doubled.
The cathode includes separators, a layer for the catalyst, a metal mesh, a hydrophobic membrane, a diffusion membrane, and a layer for air distribution. The catalyst layer contains a blend of carbon and manganese for adequate conduction, and becomes hydrophobic with the addition of teflon particles. The metal mesh gives the battery structural support while the air distribution layer permits air to enter the battery evenly over the surface.
ChemistryZinc-air batteries produce electrochemical energy by using oxygen straight from the air. Oxygen becomes the cathode reactant, and is diffused directly into the battery. The air cathode uses an aqueous alkaline electrolyte to catalytically promote the reaction of oxygen, but is not depleted or transformed at discharge. The cathode is compact, yet at the same time has an almost unlimited capacity, and achieves high energy densities due to the additional volume available for the zinc anode.
The advantages of a zinc-air battery include flat discharge voltage, safety and environmental benefits, good shelf life, and low cost. In addition, zinc-air batteries have high volumetric energy density compared to most primary batteries. The disadvantages of such batteries are that they rely on ambient conditions, they dry out once exposed to outside air, they have flooding potential, they have limited output, and their active life is short. It is important to note that when zinc turns it into zinc oxide it expands, and there must be adequate space within the battery for this expansion. The main form of gas transfer degradation is water vapor transfer.
Additional InformationToday, zinc-air batteries are used for such things as hearing aids, pagers, and medical devices. Zinc air batteries come in many shapes and sizes. They have an open-circuit voltage of 1.4 volts, and 1.15-1.35 at 20 degrees Celsius. The efficient cathode of these batteries, first developed during fuel-cell research for the space program, has since evolved with fluorocarbons becoming a polymer class.
Many variables affect power, including the type of separator system, electrolyte, cathode catalyst, and regulation of gas diffusion. Zinc-air batteries can be made for high rate applications, which have a short life but high output, or low rate, with low power but lasting a long time. Gas diffusion must be regulated to control environmental tolerance versus the demand for power.