
Thermal Batteries
Thermal batteries belong to the primary reserve battery group and use inorganic salt electrolytes (non-conductive solids at ambient temperatures) which require materials to melt them.
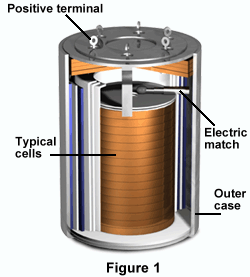
An energy impulse from an external source activates the thermal battery and ignites pyrotechnic materials within the battery to melt the electrolyte. In turn, the battery becomes conductive and produces high power for a short period of time (from a few seconds to an hour). The exact lifespan of individual thermal batteries depends on both design and application.
ChemistryThermal batteries have an alkali or alkaline earth metal anode, a salt electrolyte, a metal salt cathode, and a heat source that is usually positioned between the cells. The most common anode for thermal batteries is lithium, though magnesium metal and calcium are also used. Lithium metal anodes use a molten lithium binder, which is pressed into a foil and cut into pieces corresponding to cell size. The foil pieces are put into an iron foil cup, which is also the electron collector. In addition, the cup helps stop lithium from migrating, which could potentially short-circuit the battery.
Thermal batteries often employ a molten eutectic mix of lithium chloride and potassium chloride for the electrolyte. Halide mixtures are preferred over sodium salts due to their lower melting points. Several cathodes can be used in thermal batteries, including calcium chromate, potassium dichromate, potassium chromate, lead chromate, metal oxides, and sulfides. In addition, thermal insulation is positioned at both ends of the cell stack, and the battery is designed to remain hermetically sealed throughout its service life.
Thermal batteries, especially calcium chromate, calcium/potassium dichromate, and calcium/lead chromate, depend on very different chemical properties. The lithium/iron disulfide has several advantages over the other types, including tolerance of discharge conditions from open circuit to high current densities, large current capability, high predictable performance, a simple construction, tolerance to processing variations, and stability in extreme dynamic environments. Therefore, this type of thermal battery is the most widely used. The lithium/cobalt disulfide battery yields better stability at high temperatures due to cobalt disulfide being stable to 650 degrees Celsius. In the calcium/calcium chromate battery, both a chemical and electrochemical reaction must take place. This battery is used less and less, as more stable forms of the thermal battery are increasingly used.
Additional InformationThermal batteries have several advantages over other batteries. Their shelf life is longer than ten years without degradation in performance. They can be activated instantly to provide power within fractions of a second, and their high peak-power density exceeds 10 watts per square centimeter. Thermal batteries are resistant to harsh environments, operate at many temperatures, are reliable after long-term storage, and require no maintenance. They are hermetically sealed, so they do not outgas, and most importantly, they are custom designed for acute voltage, start time, and configuration requirements. Their disadvantages include a very short activated life (usually under 10 minutes), low energy density, a surface temperature of 230 degrees Celsius or higher, nonlinear voltage, and a one-time usage.