
Solid-Electrolyte Batteries
Unlike more common batteries, solid-electrolyte batteries contain a solid rather than liquid electrolyte.
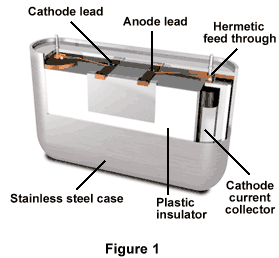
The illustration shows that the solid-electrolyte battery has a case-neutral design, contains a stainless-steel housing, and has a plastic insulator on the inside of the case (Figure 1). A lithium envelope within the plastic serves as the anode, and also contains the battery's depolarizer. The case has several hermetic "feed-throughs" that allow the current collector leads from both the anode and cathode to pass, and they are positioned in the center of the cell. Both the cathode and anode leads are on the top of the battery.
ChemistryApplying significant pressures to the cathode, electrolyte, and anode, forcing them into a circular disk form. The cells must be held at spring pressure within a stainless-steel container. Another feature of the solid-electrolyte battery includes a case-positive design.
The development of the solid-electrolyte (rather than a liquid electrolyte) battery began because scientists recognized the potential that solids could be electronically insulating elements with low ionic resistance, and could eventually be used as the electrolyte in a battery. Solid-electrolyte batteries function well at normal temperatures, and are used to power objects such as heart pacemakers and virtual computer memory. They rely on small amounts of power and a long shelf life, and have gone from a pellet-based architecture with thick layers of electrolyte to thin film cells with infinitesimal thickness. This reduction in inter-electrode distance overcomes ohm losses of earlier sold electrolyte cells. There is no loss of capacity after one year of storage, at 20, 45, and 60 degrees Celsius. Today, the solid-electrolyte battery serves as an appropriate means for providing backup power, especially for volatile computer memory.
Commercial solid-electrolyte batteries utilize lithium anodes. Lithium makes a good anode because it contains high specific capacity on a weight and volume basis. It is also electropositive, which means it has the potential for high voltages. Appropriate lithium ion conductors may be employed as solid electrolytes.
Additional InformationSolid-state batteries do have advantages over liquid components, including a very high thermal stability, little self-discharge, and the ability to function in a high range of environmental conditions. They have high energy densities, good storage life, and no leakage, and their disadvantages include low power, mechanical stress, and reduced electrode efficiencies. Currently, the only commercial solid-electrolyte battery is the Li/LiI/I2 (P2VP).
Solid electrolyte batteries contain a long shelf life, sustain low power, do not gas as a result of a hermetic seal, operate from -140 degrees to 170 degrees C for pure lithium anodes, and up to 300 degrees C for compound anodes. They also have high volumetric density, and are suggested for low-rate applications. Discharge properties of these batteries result in high energy densities, low-rate capability, and low self-discharge. People must be careful to prevent short-circuiting of the solid-electrolyte battery, which could drain the cell.