
Vented Sintered-Plate Nickel-Cadmium Batteries
The vented sintered-plate nickel-cadmium battery, a more advanced version of the nickel-cadmium battery, is another type of secondary battery (Figure 1).
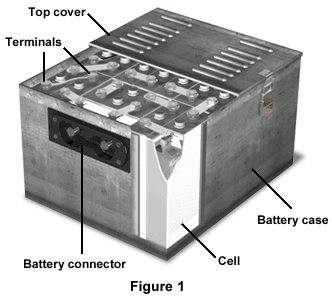
The top cover, complete with vents, covers the battery, and attaches to the battery case. Beneath the top cover are various flat and curved terminals, spacer kits, and spacers. The battery itself is made up of 20 individual cells, and the battery connector is located on the front side of the battery case.
ChemistryVented sintered-plate nickel-cadmium batteries use both flat positive nickel hydroxide and negative cadmium plates. Materials that serve as gas barriers and separators isolate the plates. Usually, the electrolyte is a 31% potassium hydroxide solution. Vented sintered-plate nickel-cadmium batteries are often called flooded cells because the electrolyte solution completely covers the plates and separators. The battery's active materials are held inside pores within its structure.
ConstructionAfter discharge occurs in vented sintered-plate nickel-cadmium batteries, both electrodes are charged at the same time. If the gas barrier fails and gas gets to the negative electrode, it will generate heat, which prevents the negative from obtaining a full charge. This results in reduced voltage as the cadmium electrode depolarizes. When assembling the battery, adequate precautions should be taken so that oxygen does not recombine at the negative plate. There are several different types of plate designs used in vented sintered-plate nickel cadmium batteries. They include substrate, plaque, impregnation, and plate formation, specific to the type of application.
The vented sintered-plate nickel cadmium battery is recharged after discharge. This recharge has four main goals. The first goal is to restore the charge to the battery as fast as possible. Second, the battery must maintain the charged capacity when the battery is removed for procedures such as maintenance. Third, the amount of water usage at overcharge must be minimized, and fourth, the damaging effects of overcharge must be reduced to the fullest extent possible.
Furthermore, as mentioned above, the battery must have periodic maintenance. Careful attention must be paid to make sure the pre-selected maintenance period schedule is assessed, and electrical performance, both capacity and power level, must be restored. Cell failures must be detected and isolated, and corresponding parts replaced. The battery must also be cleaned, its electrolyte's water replenished, and charging system calibrated.
Additionally, some vented sintered-plate nickel-cadmium batteries have cooling and heating systems, as well as temperature sensors. The cases of these batteries are usually made from stainless steel or steel with a protective finish, as well as other materials with resistance to damage from potassium hydroxide.
Additional InformationThe sintered-plate nickel-cadmium battery, a newer version of the nickel-cadmium battery, performs at a 50% increase in energy density over its predecessor. The cell has lower resistance because the sintered plate is much thinner, and therefore works well in low temperatures. The battery performs better than other batteries at various discharge loads and temperatures. It needs little maintenance, is reliable, mechanically sound, rechargeable, and has a long storage life. Sintered-plate nickel-cadmium batteries are used for high-power discharge applications like aircraft turbine engines and diesel engines, as well as mobile military equipment.