
Metal/Air Batteries
In recent years, emphasis has been placed on the metal/air battery because of its high energy density and potential long shelf life.
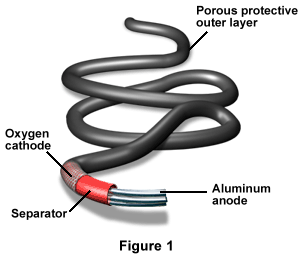
Figure 1 shows a rope battery, which is a type of aluminum/air battery. At its tip is the aluminum anode, followed by the separator, oxygen cathode, and protective outer layer.
Metal/air batteries are characterized by a high energy density, a flat discharge voltage and long shelf life. They are environmentally safe when properly disposed and available at a relatively low cost. In the past, larger metal/air batteries using zinc were used in railroad applications, communications, and other applications requiring long life and a low rate of battery discharge. Smaller batteries of this type are used in items such as pagers and hearing aids. Zinc is an excellent metal for use in a metal/air battery because of its stable alkaline electrolytes. Metal/air batteries either use alkaline or neutral electrolytes. Other metals such as lithium, calcium, magnesium, aluminum, or iron may be used in metal/air batteries.
In metal/air batteries, the reactive anode and air electrode result in an inexhaustible cathode reactant. Ampere-hour capacity in the anode, as well as the handling and storage of reaction products determines capacity limit. There are primary, reserve, and both electrically and mechanically rechargeable metal/air batteries. While the mechanically rechargeable battery is much like a primary battery, the electrically rechargeable type needs a third or bifunctional electrode for oxygen evolution.
The performance of these batteries can be affected in several ways. Limitations in the oxygen or air electrode of metal/air batteries cause voltage to decrease sharply with increasing current; therefore, these batteries are appropriate for applications that require low to moderate power. Carbon dioxide can be absorbed by the battery, which may lead to carbonate crystallization in the air electrode, decreased performance, and may cause damage. Systems recharged electrically allow the oxidation of catalysts and electrode supports during charging. In addition, an effective air electrode must be utilized for adequate performance.
Zinc/Air BatteriesZinc/air batteries come in both primary button cells and larger cells for industrial and smaller applications. The issues of controlling the recharge of the zinc, as well as a bifunctional air electrode, need to be perfected to have an electrically rechargeable zinc/air battery. There are portable primary zinc/air batteries, industrial primary zinc/air batteries, and primary hybrid-air/manganese dioxide batteries, which use a hybrid cathode containing manganese dioxide. There are also electrically rechargeable zinc/air batteries that use a bifunctional oxygen electrode for charge and discharge, and mechanically rechargeable zinc/air batteries that require the replacement of discharged anodes.
Aluminum-Air BatteriesAluminum is a good metal for metal/air batteries because of its ampere-hour capacity, specific energy, and voltage. There are aluminum/air batteries using neutral electrolytes for portable equipment and marine applications, and those using alkaline electrolytes for things like emergency power supplies and field-portable batteries, as well as underwater transportation.
In addition, two other popular types of metal/air batteries are magnesium/air and lithium/air. Out of all anodes that apply to a practical battery system, lithium has the highest theoretical voltage, and is a desirable choice for underwater batteries.