
Ambient-Temperature Lithium Anode Reserve Batteries
Frequently used as a reserve battery anode, lithium's high potential and low equivalent weight enables it to provide large amounts of energy.
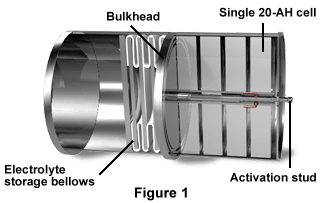
Figure 1 depicts a cross section of the 20-AH lithium/sulfur dioxide multi-cell battery. The individual cells are positioned between the bulkheads (below) and the storage bellows (above). Between the rows of cells and down the center of half the battery is the activation stud. A lithium reserve battery operates at twice the voltage of aqueous battery types, and because lithium is so reactive to aqueous solutions, it usually uses a non-aqueous electrolyte. In a reserve battery configuration, the active electrode materials and electrolyte are physically separated.
One strong property of the reserve design is that the battery has undiminished output after long periods of storage (over fourteen years). When building a lithium anode electrochemical system as a reserve battery, one must consider electrolyte stability, as well as compatibility between electrolyte and the materials used in the electrolyte reservoir. There are three major types of ambient-temperature lithium anode reserve batteries: Lithium/vanadium pentoxide, lithium/thionyl chloride, and lithium/sulfur dioxide.
Lithium/Vanadium Pentoxide BatteryThis battery has a lithium anode, a microporous polypropylene film separator, and a vanadium pentoxide and graphite cathode. The voltage level at first is 3.4 to 3.3 volts, which lowers to 3.3-3.2 volts, and finally decreases to 3.2-3.1 volts. The storage capability depends on the electrolyte solution's stability.
Lithium/Thionyl Chloride BatteryIn this system, the anode is lithium, the separator is a non-woven glass, and the cathode is a teflon-bonded carbon medium. Thionyl chloride provides both the solvent and active cathode materials. The electrolyte for this battery is extremely stable and has excellent performance.
Lithium/Sulfur Dioxide BatteryThis battery also contains a lithium anode, a separator, and a cathode of teflonated carbon. The electrolyte solution also serves as the active cathode material and has a blend of lithium bromide, acetonitrile, and sulfur dioxide. One disadvantage of this battery is that it is not stable during storage.
Lithium anode reserve batteries have many unique characteristics. They operate at temperatures between -55 and 70 degrees Celsius, with a 10 to 20 year inactivated storage life, and must be hermetically sealed. They have superior energy density, are reliable, have little electrical noise, contain fast voltage rise after initiation, and offer a wide range of service life, from several seconds up to a year.
Additional InformationThe design of ambient-temperature lithium anode reserve batteries varies by application. They come in simple, single cell sizes with a manually activated ampoule, to large, complex, multi-cell batteries that are initiated automatically. The case for these batteries is usually composed of stainless steel to protect against corrosion, the terminals made of glass or metal, and the battery is kept hermetically sealed. Three types of basic lithium reserve batteries are manufactured: the single-cell battery in which a glass ampoule stores the electrolyte, multiple single cells whose storage reservoir uses bellows, and multi-cell or bipolar construction with a glass ampoule or metal reservoir to store the electrolyte.