
Lithium Batteries
Lithium makes a good anode material because it is lightweight, capable of high voltage, contains high electrochemical equivalence, and is a good conductor. One advanced lithium battery, the lithium/sulfur dioxide battery, has many military and industrial applications.
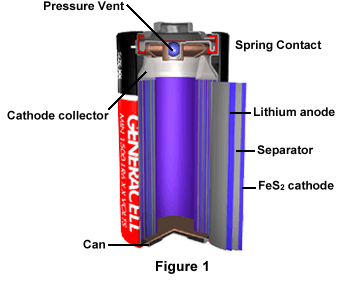
As illustrated in Figure 1, the lithium/iron sulfide battery uses an anode of lithium foil and iron sulfide as the active material in a porous carbon cathode. A non-aqueous electrolyte such as an organic solvent blend is used, and the anode, cathode, and electrolyte combine for high current and low temperature performance. The battery, which activates around 90 degrees Celsius, contains a vent to release high pressure and there is a remittable terminal switch located at the top.
Types of Lithium BatteriesThere are several different categories of lithium batteries based on the type of electrolyte employed. Soluble-cathode batteries use liquid or gaseous cathode materials such as sulfur dioxide or thionyl chloride. These substances form an essential protective coating that prevents self-discharge between the anode and cathode. Two types of soluble-cathode lithium primary cells are currently in production. One uses sulfur dioxide as the active cathode, and the other utilizes an organic solvent, usually an oxychloride. Solid-cathode batteries, as the name implies, use a solid cathode and do not require hermetic seals. Some types of this battery include a lithium and manganese dioxide, lithium/carbon monofluoride, lithium/copper oxyphosphate, and lithium/silver chromate. Solid electrolyte cells have long storage lives, but low discharge rates.
General InformationThe advantages of lithium batteries include high voltage (up to 4 volts), high energy density, operation over a wide temperature range, good shelf life, and excellent power density. Because lithium reacts to aqueous solutions, non-aqueous solvents for the electrolyte must be used. Therefore, lithium battery electrolytes must be non-aqueous such as polar organic liquids, which are the most common solvents for active primary batteries (but not in thionyl chloride and sulfur chloride cells.) Furthermore, the electrolyte must be apriotic, or contain no protons or hydrogen atoms (though it may contain hydrogen molecules) that will react with lithium, must form an ionically conductive electrolyte, and must remain a liquid at most temperatures.
For these reasons, lithium batteries remain the standard form of battery production for high performance primary and secondary batteries over the last ten years. Their development has been going on since the 1960’s, concentrating on non-aqueous batteries using lithium anodes. In the early 1970’s, lithium batteries were used in military applications, but only on a limited basis largely due to safety considerations, formulations, and cell structures. Lithium batteries now come in a wide range of sizes and shapes. As scientists find ways to conquer the safety hazards of lithium batteries, many new application opportunities will open up in the future. For now, careful attention must be given to prevent physical and electrical abuse of lithium batteries, and to ensure safe operation and proper disposal -- an important consideration since some lithium batteries are flammable and/or explosive.