
Lead-Acid Batteries
The lead-acid battery is a reliable battery system that operates within a large temperature range, and its charge-discharge process is practically reversible.
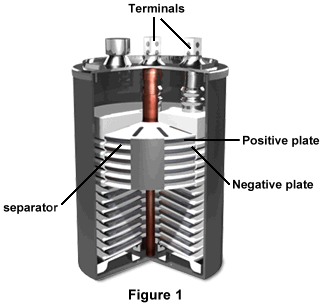
Figure 1 displays a cutaway of a lead-acid battery. The negative and positive plates are isolated from each other by a separator, and several stacks of these plates makes up the battery. The base of the battery is also plate-shaped, and contains both a plug and a nut. The positive and negative terminals appear at the top.
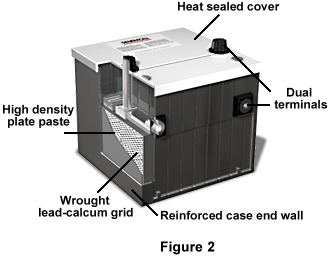
Figure 2 displays a more commonly known lead-acid battery, the typical car battery. The covers are heat sealed to prevent electrolyte contamination, and there is a liquid-gas separator area, which returns liquid to the reservoir, thus providing the battery with a longer life. The battery also has high-density plate paste for higher output, and a "small window" lead-calcium grid. It has dual terminals for universal application, separator envelopes, and a reinforced case end wall.
ChemistryIn the lead-acid battery, the active material within the positive electrode consists of lead dioxide, while the negative active material is a metallic lead. The positive active material is formed electrochemically from a cured plate, and influences the performance of the lead-acid battery. The electrolyte consists of a sulfuric acid solution, and as the battery discharges, the electrodes are converted into lead sulfate, which reverses when the battery is charged. The nominal voltage of this battery cell is 2 volts.
ConstructionThe major component in the lead-acid battery is highly purified lead, from which alloys are made so that the lead can be made into a grid-like material, as lead in its pure form is unable to maintain this shape. The lead alloy is fashioned into a grid to hold the active material mechanically, and to conduct electricity between this material and the terminals of the battery. Calcium, silver, and cobalt are among many materials that can be used to make the lead stiffer. Lead oxide is made into a dough-like material so it can be attached to the grid. The lead-acid battery uses three types of positive plates: tubular and pasted flat plates for automotive power, stationary applications, and diesel engine cranking, and Plante designs, where the active material consists of pure lead.
Additional InformationNew applications for battery power in fields such as storage, emergency power, and electric vehicles, as well as the more traditional uses in automobiles, boats and planes, warrants the continued dominant use of lead-acid batteries. These batteries are responsible for about half of all battery sales. The low price and typically local production of these systems makes them easy to use extensively and the least expensive storage battery.
The lead-acid battery has several advantages. It is a secondary battery with a low cost, with varying rates of production worldwide. Therefore, there are a lot of these batteries around the world, and their sizes range generally from 1 ampere-hour to several thousand ampere-hours. The battery performs well at a high rate, which makes it especially good for starting engines. It has a turnaround efficiency of over 70%, and a high cell voltage of over 2 volts, which is the highest of aqueous-electrolyte battery systems.
It has several disadvantages as well, including a low cycle life, which is about 50-100 cycles for the average lead-acid battery. It also has a limited energy density and poor charge retention. Long-term storage of lead acid batteries in a discharged condition can cause sulfation, or a polarization of electrodes. The battery is difficult to make in very small sizes, can at times be an explosion hazard, and when improperly disposed, may create environmental risks.