
Lithium/Iron Sulfide Batteries
Lithium alloy/metal sulfide batteries use an electrolyte made of molten salt and solid, porous electrodes, operating in a temperature range of 375-500 Celsius depending on electrolyte composition. If molten salt electrolytes are used, high fast-electrode kinetics and high electrolyte conductivities result in the battery's high power density. Impedance is reduced because of its bipolar design, which therefore enhances the power.
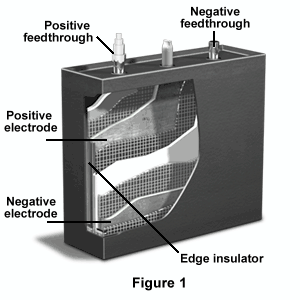
Figure 1 shows a cutaway view of a lithium-aluminum/iron sulfide battery. Both the positive and negative feedthroughs appear on the top of the battery, along with an electrolyte fill tube. Both the negative and positive electrodes can be seen on the inside of the battery, as well as the felt separator, edge insulator, and edge overlap channel.
Lithium with aluminum or silicon alloys are employed as reversible solid negative electrodes. Iron, nickel, cobalt, or other select metal sulfides are often used as negative electrodes because they reduce lithium activity and result in large coulombic efficiencies.
These batteries have both high power and high energy densities, are resistant to overcharge, over-discharge, and freeze-and-thaw. They are also relatively safe, are reliable, work well in most environmental conditions, and contain a capacity independent of load. However, the battery needs a thermal management system to maintain appropriate temperature.
Originally, the cells of lithium/iron sulfide batteries were more like automotive lead-acid batteries since they employed a prismatic cell design. The multi-cell plate maintains appropriate ampere-hour capacity by containing flat-plate positive and negative electrodes, which are separated by porous sheets. The negative electrodes are attached to the cell case while the positive ones are connected to a terminal outside of the cell case. The battery can be put together in a charged, uncharged, or partially charged condition.
Bipolar lithium/iron batteries are being used for automotive electric vehicle and pulse power projects in the United States and submarine applications in Great Britain. The batteries have the potential to be miniaturized due to how compact they are, as well as their ability to work well in many different temperatures. Lithium activity of the lithium aluminum anode, as well as the rate of transport of dissolved lithium, affect the battery's self-discharge rate.
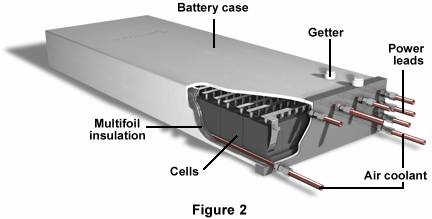
Figure 2 shows a lithium-aluminum iron sulfide electric van battery. Beneath the case, one can see the heat exchanger, flexible connectors, insulation, and the cells themselves. Just under the case is some multifoil insulation, and on the case are the power leads, air coolants, instrumentation leads, and a getter.

Figure 3 shows an electric vehicle battery, which has 36 volt stacks combined with multifoil insulating plugs on the inside. The side displays instrumentation and control, heater power, negative bus, getter heater, and just inside the side is the thermally activated getter.