
Ammonia Batteries
Ammonia batteries include a family of batteries that use an ammonia electrolyte solvent. They were originally constructed to satisfy the need for a long storage, low-powered battery with an operating life of several weeks.
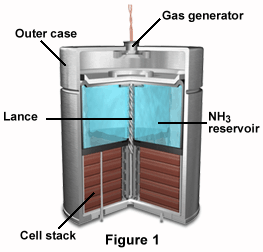
The ammonia battery illustrated in Figure 1 uses the magnesium/meta-dinitrobenzene (Mg/m-DNB) system. It has a cylinder-shaped outer case, a gas generator, and an ammonia reservoir in its center.
ChemistryThe ammonia battery includes an anode, a cathode, electrolyte, and a separator that prevents the different electrodes from contacting each other. Electrolyte salts are placed on the separator, and/or in the cathode when the battery is first built, and the salts become conductive only when ammonia enters the battery and the salts dissolve. The positive terminal, or cathode terminal, uses a metallic plate as its conductor. Often, the anode is made of magnesium while the separator consists of filter paper and paper pulp or glass fiber with added carbon to make the binder and conductor for the active cathode material.
There are several types of electrochemical couples that function in ammonia-solvent electrolytes. The most popular of these is the Mg/m-DNB batteries, where the anode consists of magnesium, the cathode uses a meta-dinitrobenzene active ingredient, and the electrolyte salt system has ammonium thicyanate (NH4SCN) and potassium thicyanate (KSCN). The dry salts are kept in the electrode stack. KSCN, a neutral salt, is positioned in the separator. The cathode itself consists of pads that press paper pulp, carbon, m-DNB, and NH4SCN together. NH4SCN ensures ionic conductivity and provides an acid environment to enhance anode activity, as well as reducing the ammonia's vapor pressure.
Additional InformationEarly in the development of ammonia batteries, scientists found that they have a poor wet-stand life; therefore, research focused on reserve battery configurations. In this design, dry electrolyte salts separate the electrodes, and the ammonia vapor in the electrode compartment activates the battery. Advantages of the ammonia battery were arming and fusing applications at a good low temperature performance. The first ammonia vapor batteries had slow activation, and scientists began to work on liquid ammonia reserve batteries.
Ammonia batteries have pros and cons. Their wet cell life is limited because the ammonia electrolyte is corrosive-- for this reason, ammonia batteries are usually reserve batteries. Ammonia batteries last in excess of 10 years on the shelf if they are not activated, and can withstand normal temperatures of -70 to 74 degrees Celsius. Liquid ammonia has a low viscosity, so activation times are enhanced. Ammonia batteries operate anywhere between -55 and 74 degrees Celsius due to the high conductivity of ammonia electrolytes. Because of excessive internal pressure, ammonia batteries require hermetic seals. Depending on the type of ammonia battery, voltage can be anywhere between 1.1 to 3.0 volts per cell.
Mass-produced ammonia batteries are always in a flat-plate configuration. Inside the battery, a plastic cup houses the cell stack to prevent short-circuiting to the battery's steel case. A tight seal is formed between individual cells to prohibit electrolyte leakage.