A PHOTOMICROGRAPHY PRIMER
It's worth a closer look
Light microscopy has classically been viewed as an experimental tool for the biological and medical sciences. In this respect, the microscope has proven useful in countless investigations into the mysteries of life. However, as lens and coatings technologies have improved over the years, visible light microscopy has slowly found applications in such diverse disciplines as chemistry, geology, physics, materials sciences, and even the semiconductor and computer industries.

|
Above is a photomicrograph of the pesticide DDT. |
Most biological microscopes are equipped only with brightfield and darkfield illumination for examining pre-stained or live specimens. To enhance contrast of unstained samples, many of the more modern microscopes are also equipped with phase contrast optics. These optical techniques are generally of little use for imaging specimens in the physical sciences where alternative methods such as light plane cross-polarization, differential interference contrast, and Rheinberg illumination are commonly employed. Unfortunately, these alternative optical illumination techniques add considerably to the initial expense when a microscope is purchased. In this article, I discuss how low-cost methods can be implemented to convert the biological microscopes found in most high schools into polarized light microscopes useful for instructional purposes in the physical sciences. Also, the basic principles of photomicrography are discussed.
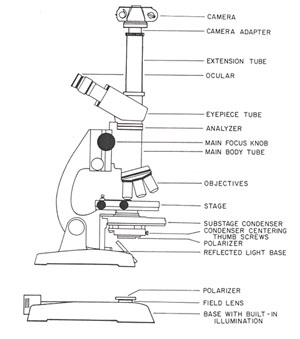
MICROSCOPE SET-UP
Several science supply distributors offer an excellent selection of high-quality optical components at reasonable prices (see the list in Figure 1). Polarizers suitable for performing crossed-polarized light microscopy can be purchased from these dealers for less than $25.00 and adapters which couple most cameras to the microscope are available for $15.00 to $100.00. Expensive high-powered microscope objectives (lenses) have a very narrow depth-of-field and are not really useful for a majority of work in the physical sciences, therefore lower cost 5x and 10x objectives, which usually come as standard equipment on most microscopes, are sufficient.
Two polarizers will be needed to convert the microscope. The first polarizer is inserted into the lightpath at the base of the substage condenser (see Figure 2). This polarizer can be held in place with tape, or if the microscope has a built-in light source, the polarizer can be placed directly over the field lens. The second polarizer, commonly termed the analyzer, is place inside the body of the microscope between the main body tube and the eyepiece tube. There is usually a lens mount at the top of the body tube and the analyzer can be placed directly on this mount. Because of the restricted space within the main body tube, the analyzer must be limited in size to 1-2 centimeters in diameter. An analyzer of the proper size can be acquired by cutting a piece of polarized sheet plastic or buying a small polarizer from a dealer (Figure 1). After installation of the polarizer and analyzer, the microscope illumination is turned on and the polarizer at the microscope base is rotated until the viewfield becomes very dark (total extinction).
Figure 1. Microscope and accessory manufactures and distributors
OLYMPUS CORPORATION
4 Nevada Drive
Lake Success, N.Y. 11042
Telephone: 516-488-3880
CAROLINA BIOLOGICAL
SUPPLY COMPANY
2700 York Road
Burlington, N.C.
Telephone:800-334-5551
E. LEITZ, INC.
24 Link Drive
Rockleigh, N. J. 07647
Telephone: 201-767-1100
CARL ZEISS, INC.
One Ziess Drive
Thornwood, N.Y. 10594
Telephone: 914-747-1800
NIKON INC. INSTRUMENT GROUP
623 Stewart Ave.
Garden City, N. Y. 11530
Telephone 516-222-0200
AO SCIENTIFIC INSTRUMENTS
P.O. Box 123
Buffalo, N. Y. 14240
FISHER SCIENTIFIC
50 Fadem Road
Springfield, N. J. 07081
Telephone: 201-379-1400
EXCEL TECHNOLOGIES
90 Phoenix Avenue
Enfield, Ct. 06082
EDMUND SCIENTIFIC CO.
101 E. Gloucester Pike
Barrington, N. J. 08007
Telephone: 609-573-6250
McCRONE ACCESSORIES AND COMPONENTS
850 Pasquinelli Dr.
Westmont, IL 60559
Telephone 312-887-7100
VVVR SCIENTIFIC
P.O. Box 330348
Houston, TX 77233
Telephone 800-392-3338
Telephone800-527-1576
At this point, the polarization direction is perpendicular between the polarizer and the analyzer and you then have what is termed crossed polarizers. When purchasing polarizers, it is important to select polarizing materials that are very close to a neutral gray in color such as the threaded polarizers made for the front of a camera lens. Avoid polarizing materials that are green or amber in color as these will not produce total extinction. Some microscope manufacturers offer a low-budget polarization kit ($150.00 to $300.00) which is easily installed. It is advisable to contact your microscope's distributor on the availability of these items if your budget allows.
The adjustments described above apply only to transmitted light microscopy where polarized visible light passes through the sample. An alternative method of microscopy utilizes reflected light where a beam of light is reflected off the surface of the sample to be examined. To avoid investing in expensive reflected light attachments, oblique illumination from an external light source can be substituted to achieve a reflected light effect. A high-intensity light source such as a fiber optics lamp provides an excellent substitute.
Attaching a camera to the microscope is the last step. Microscope viewing heads come in three varieties: monocular (one eyepiece), binocular (two eyepieces), and trinocular (two eyepieces and a photography tube). A camera can be adapted to each of these viewing heads. Commercial aftermarket camera adapters usually are attached to one of the viewing tubes with a thumbscrew and adjusted to be parfocal with the eyepieces by sliding the adapter up or down on the viewing tube. A simple camera back will be sufficient for photomicrography because the camera is only required to store, expose, and advance the film. The microscope itself acts as the camera lens.
For photomicrography, it is very important to ensure that your microscope is aligned to produce even illumination across the viewfield. Information on microscope alignment is available in the owners manuals or in textbooks dealing with microscopy.
|
|
SAMPLE PREPARATION
The Laboratory chemicals found in high school chemistry stockrooms provide an excellent source for samples. The chemicals listed in Figure 3 are easily obtained and are known to produce good crystals for viewing with a polarized light microscope. Most crystals are anisotropic and birefringent, which means that will refract plane-polarized light emitted from the polarizer and will "bend" it until it is visible through the analyzer under crosspolarized illumination.
To prepare crystals for examination in the microscope, deposit a few milligrams of the appropriate chemical on a glass microscope slide and carefully place a glass coverslip over the powder. Next, heat the bottom side of the microscope slide carefully with a bunsen burner or hot plate until the powder has completely melted. (NOTE: Some chemicals decompose upon heating and will provide poor subjects for microscopic examination. Others produce harmful vapors and should be avoided.) When molten, the chemical will flow underneath the coverslip and fill the entire volume between the coverslip and the microscope slide. Either allow the slide to cool slowly before examination, or place the melted chemical on the microscope stage and examine the crystallization process as it occurs.
Certain chemicals recrystallize very rapidly (within a few minutes) while others may recrystallize slowly over a period of days, weeks, or even months. Urea and benzoic acid are excellent examples of common laboratory chemicals that will recrystallize rapidly enough to be examined directly after melting. The instructor and students should experiment with safe, familiar chemicals that are available to identify those that are optimal for microscopic analysis.
Another very effective method of preparing crystals is to dissolve the chemical in a suitable solvent such as water, ethanol, or mineral spirits. A drop of the solution is sandwiched between the microscope slide and coverslip and the solvent slowly allowed to evaporate, resulting in formation of crystalline patterns. This method is especially useful for chemicals in the salt family that usually decompose upon heating and leave a tar-like mess. Chemicals can display a wide spectrum of polymorphic crystalline patterns depending on whether they are melt-recrystallized or recrystallized by solution evaporation.
CLASSICAL PHOTOGRAPHY ASSIGNMENTS CAN BE COUPLED WITH SCIENCE MICROSCOPY STUDIES TO PROVIDE A MULTIDISCIPLINARY PROGRAM IN PHOTOMICROGRAPHY
Samples for reflected light microscopy usually require very little preparation. Reflected light microscopy can be likened to topographical surface examination with a high-power magnifying glass, and almost anything can be examined in microscopic detail with this technique. For example, the fine details of surface structure can be revealed on leaves, coins, printed paper, insects, and a variety of other specimens.

|
Lanthanum Aluminate is a substrate for the thin film deposition of superconducters. The stair-step twinning in the photo interferes with coherent film formation. |
Perhaps the most interesting subjects for reflected light examination are integrated circuits. These electronic "chips" generally are packaged either by being molded into plastic cases or cemented into ceramic cases. It is virtually impossible to recover an integrated circuit from a plastic case because the epoxy resin flows into the microstructure on the circuit surface and cannot be easily removed. However, the cement that secures the two halves of an integrated circuit ceramic case can be scored with a hacksaw and split with a fine chisel to reveal the internal chip. The chip is cemented into a depression on the bottom section of the ceramic case and can be viewed directly without removal from the case. Leaving this portion of the casing intact also serves to protect the delicate silicon surface of the integrated circuit. Most programmable read-only memory (E-PROM), random access memory (RAM), microprocessors, and many other digital and analog integrated circuits are protected with ceramic cases. Defective integrated circuits are quite satisfactory for examination because the defect is usually not apparent on the surface of the circuit.
An excellent source for non-working integrated circuits is computer or electronics repair shops. These shops normally stockpile a large quantity of defective integrated circuits and will usually give them away at no cost. Alternatively, many new integrated circuits are available from dealers for less than $2. Be sure to specify that you require ceramic cases when ordering new integrated circuits.
Examination of integrated circuits with reflected light can serve two purposes. Details of a particular circuit structure are readily apparent and, by observing differences in the architecture of various integrated circuits, students can begin to get a handle on the complex electronics involved in modern devices such as radio, television, and computers. Reflected light microscopy has become an indispensable tool for the semiconductor industry due to its usefulness in characterizing manufacturing defects and monitoring the successive stages of integrated fabrication.
PHOTOMICROGAPHY
A necessary responsibility of microscopy is to capture the images seen in the microscope onto photographic film to obtain "hard copy" for research records. In a high school environment, classical photography assignments can be coupled with science microscopy studies to provide a multidisciplinary program in photomicrography.
Photomicrography encompasses the techniques of both black-and-white and color photography. Black-and-white film processing is substantially lower in cost than color film, if processing is done in-house. Many commercial film processors no longer offer black-and-white processing services or charge exorbitant amounts for this service. If budget restrictions force the exclusive use of black-and-white photomicrography, it is advisable to invent in darkroom equipment so students can develop and print their own photomicrographs.

|
This moon rock sample was collected by Apollo 12 astronauts from the Moon. It is 3.15 to 3.35 billion years old and provided the first indications that granite exists on the Moon. |
A green filter should be inserted into the microscope lightpath between the light source and the first polarizer (see Figure 1) for black-and-white photomicrography. I recommend the use of Kodak™ Technical pan film with HC-110 developer for crisp images with excellent resolution. Printing can be done on Kodak Polycontrast™ paper with Dektol™ developer. After processing a roll of film, carefully cut the negatives into sections of 5 frames each and store in specially made polyethylene storage sheets. You can make contact prints by placing a sheet of negatives directly onto an 8" x 10" piece of polycontrast paper and exposing for a few seconds with the enlarger lens aperture wide open. Contact sheets are an ideal way of cataloging data and they provide a compact method for storing or sorting through many images. When an enlargement is needed, simply remove the appropriate negative strip.
Color photomicrography is considerably more complicated than black-and-white photomicrography because color film emulsions are color balanced for a particular spectrum of light. The term "color temperature" refers to the wavelength spectrum emitted by a particular light source. For instance, films intended to be used outside in ordinary daylight or under fluorescent lighting are balanced during manufacture for a color temperature of 5500º K while films made for indoor tungsten light bulb use are balanced for a color temperature of 3200ºK.
The majority of microscopes use a tungsten-halide lightbulb as a light source that emits a wavelength spectrum centered in the 3200º K color temperature region. Therefore, films color balanced the best results. All major film manufacturers have one or several 3200º K films available in 35-mm transparency format. Transparency film is preferable to color negative film for several reasons: Color negative films are balanced for 5500º K and must be manipulated during printing to avoid a decidedly yellowish cast. Most photoprocessors cannot or will not produce satisfactory results with photomicrographs on color negative film. The contrast and color saturation in transparency film cannot be equaled by color negative film. Color transparencies are easier to label, store, and catalog, and they can be projected at seminars.
With a 20-to-50 watt tungsten-halide bulb in your microscope, exposure times are usually very short and allow the use of slow films such as Ektachrome 50 or Fujichrome 64T. Using slow films reduces the grain in photomicrographs. If tungsten-balanced films are not available, a Kodak 80A or equivalent filter can be inserted into the lightpath between the light source and the first polarizer to allow the use of daylight balanced films with minimal color shift. But, if this filter is used, exposure times must be increased one to three f-steps to allow for a reduction in light intensity.
COLOR PHOTOMICROGRAPHY IS CONSIDERABLY MORE COMPLICATED THAN BLACK-AND-WHITE PHOTOMICROGRAPHY
When photographing new samples or after making changes to the microscope (such as installation of polarizers), the new exposure characteristics should be determined on a test roll of film. Bracket several exposures of the same viewfield at least one and preferably two f-steps over and under previous exposure times. This will assure at least one or several good exposure times. This will assure at least one or several good exposures and will yield exposure time information useful for photomicrography of future samples.
The best film, in my opinion, is Fujichrome 64T, a highly color-saturated E-6 transparency film with excellent contrast. Recently, a new emulsion of this film was introduced that is designed to allow push processing with very little reduction in image quality. Push processing is a method developed to increase contrast (inherently low in photomicrographs) and color saturation. This is accomplished by underexposing the film one to two f-steps and increasing the process time in the first developer during the E-6 process.
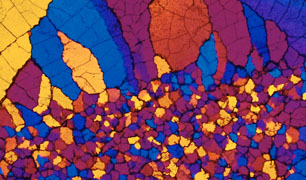
Calcium Chloride Crystallites
INDEPENDENT STUDENT RESEARCH PROJECTS
A variety of specific applications are available to teachers and students in the form of individualized student research projects. The following paragraphs should serve as an introductory guide to the myriad of projects which are possible.
1. By examining a number of different integrated circuit types, the student can become familiar with details of various electronic design motifs. For instance, microprocessor integrated circuits generally contain a ROM and RAM section for internal calculations and storage of information. These memory sections differ from one circuit to another and also from the same type of circuits provided by different manufacturers. Also, transistor size has steadily decreased as more transistors are packed onto a single integrated circuit. Many older, non-working integrated circuits are available from electronics repair facilities, and these circuits can be examined for transistor size versus manufacturer date. In addition, memory circuits can be studied to relate microscopic features to the actual storage capacity of the integrated circuits. These types of investigations should be undertaken by students who have an interest in engineering and electronics technology.
2. The crystallization patterns of a single set of biochemicals-vitamins, for instance-can be observed by assembling a collection of recrystallized biochemicals. In a large biochemical family, such as the vitamins, many different types of organic chemical groupings are presented. This leads to a large spectrum of different crystalline morphologies. By combining specific information about the individual vitamins with photomicrographs of the vitamins, the student should succeed in producing a interesting and informative photographic display. Students interested in biology, chemistry, and biochemistry would benefit from this experiment.
3. For students who are primarily interested in photography, the beautiful colors provided by polarized light microscopy can serve to sharpen color photography skills. Because the microscope has a relatively fixed setting when compared to standard photography, students can compare the results produced by different films under indentical conditions. Also, students can easily see the effect, on photomicrographs, of small differences in color processing variables.
4. The topic of art in science is becoming increasingly more popular. Students interested in this area can benefit by producing a selection of color photomicrographs and mounting them for display in local art galleries and libraries. This project will provide students with experience in photomicrography as well as the details pertaining to preparing photographs for display.
By introducing polarized light microscopy and photomicrography to high school students, you give them experience with a technique that is becoming a mainstay of modern science and industry. Students will find that their creativity in photomicrography is limited only by the boundaries of their own imagination.
Figure 3. Common chemicals suitable for recrystallization.
Alka-Seltzer - Best crystals from aqueous solution.
Ascorbic acid (vitamin C) - Can either be melt-recrystallized or recrystallized from alcohol.
Aspartame (Nutra-sweet) - Good crystals from melt-recrystallization or aqueous solution.
Aspirin (acetylsalicylic acid) - Equally good crystals from melt- recrystallization or aqueous or ethanol solutions.
Acetaminophen (Tylenol) - Equally good crystals from melt-recrystallization or aqueous solution.
Benzoic acid - Best crystals from melt-crystallization.
Biotin (vitamin H) - Best crystals from melt-cystallization.
Citric acid - Good crystals by evaporation from aqueous ethanol solutions.
Epsom salts - Recrystallize from aqueous solution only.
Glucose and sucrose (common sugars) - Crystals quickly obtained by evaporation of solution in water. Very beautiful crystals after melt-crystallization.
Ibuprofen (Advil) - Best crystals after evaporation of rubbing alcohol from ethanol.
Kodak Dektol paper developer - Best crystals after evaporation of working stock solution.
Kodak D-76 film developer - Best crystals after evaporation of working stock solution.
Kodak rapid fixer - Best crystals after evaporation of working stock solution.
Niacin (a B vitamin) - Beautiful crystals after melt-crystallization.
Nicotinic acid - Good crystals from melt-or evaporation from alcohol solutions.
Tartaric acid - Very good crystals from ethanol solutions.
Urea - Crystals form rapidly after melt-recrystallization.
NOTE
The author would like to thank the Nikon Instrument Group for providing photomicrography equipment and the FSU Center for Materials Research and Technology for continued support.
REFERENCES
Delly, J.G. 1988. Photography Through the Microscope. New York: Eastman Kodak Company publication.
Davidson, M.W., and R.L. Rill. 1989. Photomicrography: Common ground for science and art. MICROSCOPY and Analysis 4:7-12.
Davidson, M.W. 1991. Fascinating photography with a simple light microscope. PHOTOgraphic Magazine (April): 92.
ABOUT THE AUTHOR
Michael W. Davidson is a research associate at the National High Magnetic Field Laboratory and the Supercomputer Computations Research Institute in the Department of Physics at the Florida State University, Tallahassee, Florida 32306.