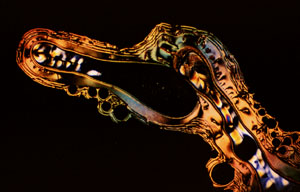
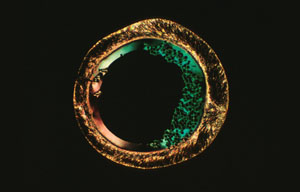
PHOTOMICROGRAPHY: COMMON GROUND FOR SCIENCE AND ART
Michael W. Davidson and
Randolph L. Rill
Department of Chemistry
and Institute of Molecular Biophysics
The Florida State University
Tallahassee, Florida 32306 USA
The escalating technology in optics and optical coatings, coupled to the development of high quality microscopes and deeply colour-saturated photographic films has, in part, led to an explosion in the utilisation of photomicrography for numerous fields. Many years ago, the microscope was an exclusive tool of biologists who spent countless hours observing and drawing various specimens of biological interest. Today, however, the microscope has found a home in disciplines as diverse as Chemistry, Physics, Geology, Psychology and Materials Science, to name a few.
|
|
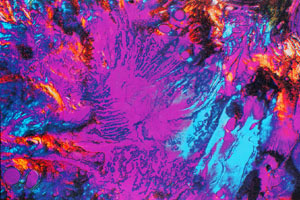
Our interest in photomicrography originated from a need to view, at relatively high magnification, the various textures formed by DNA as it entered the liquid crystalline state (illustrated in Figure 11-4). To this end, we have used both light and electron microscopy; however, this article will be dedicated to our colour photomicrography with a polarising light microscope.

|
|
In December 1986, we first observed a highly birefringent, beautifully coloured, fan-textured smectic (2-dimensionally ordered) liquid crystalline state of DNA (Figure 24) which we attempted to photograph using standard E6 transparency films processed by commercial laboratories. The results that we obtained were disappointing because the contrast and colour saturation of our transparencies were of an inferior quality to the specimens that we observed first-hand in the microscope. To correct this, we initiated a series of tests using various daylight and tungsten-balanced films which we began processing in-house with commercially available Kodak E6 kits. After many months of tests and comparisons, we finally settled on Fujichrome 64 T and have been using this film almost exclusively, with the exception of Polachrome HC 35 mm instant colour transparency film, which we employ for the annual Polaroid International Instant Photomicrograph Competition.
|
|
Our entry into the photomicrograph competition circuit started with the 1987 Nikon Small World contest in which we received 6th and 9th prizes and two honorable mentions for photomicrographs of liquid crystalline DNA samples. The Nikon contest heralded a new era for our photomicrography because one of the winning micrographs was a multiple exposure which we intended to resemble an alien landscape. Photomicrographs of this type have now won many competitions5 and we have extended our emphasis to make these photographs more life-like. We now term these types of photomicrographs, Microscapes. Several examples of our most recently fabricated Microscapes are illustrated in Figures 3-12.

|
|
Fabrication of Microscapes
Nikon's UFX-II digitally controlled exposure monitor allows for a double or multiple exposure mode which is employed in the construction of multiple exposure Microscapes. Our microscope is a Nikon Optiphot-pol in which light intensity and distribution are regulated by the field diaphragm (a leaf-type shutter) and emitted through a lens in the base of the microscope. By carefully masking a portion of the field diaphragm lens, selective areas of film can be exposed to light captured through the microscope.
|
|
The sun or moon is added by closing the field diaphragm almost completely and defocusing the image until the individual leaves merge to form a complete circle. Next, an orange or red filter is inserted into the light pathway and the substage condenser is decentered, by realignment of the adjustable centering pins, to move the diaphragm image to the appropriate location and an exposure is recorded. Long exposures yield a bright, white centered sun or moon, while short exposures give more colour-saturated images with a yellow-to-red transition from centre to edges. The new moon illustrated in Figure 12 is created by placing the tip of a ball-point pen in the light path after closing down the field diaphragm and defocusing.
|
|
The mountains are masked exposures of unrefined Xanthan-gum dissolved in aqueous solution and allowed to concentrate while sandwiched between a microscope slide and a coverslip. This polysaccharide tends to undergo a transition to cholesteric liquid crystalline states, in concentrated solutions, which resemble mountainous formations.
|
|
The morning sky is created by cutting a rectangular portion of a polythylene film (plastic storage bag, thickness~100 micrometers) and stretching it in a uniform manner before pressing onto a microscope slide. Upon stretching, the polyethylene molecules align to a form birefringent diffraction gradient which casts a yellow-to-red-to-blue visible light spectrum to simulate a morning sky. After masking previous exposures, the film is exposed to a masked section of the diffraction spectrum in order to obtain the effect desired.

|
|
Stars are created by imaging small cholesteric liquid crystalline spherulites of polybenzyl-1-glutamate dissolved in dimethyl formamide. Previously exposed areas of the film are masked for these exposures in order to eliminate burning of the spherulite image.
|
|
The clouds present in Figure 9 are generated by defocusing colourless birefringent crystals of Cibachrome bleach.
|
|
Various foregrounds are obtained by photographing crystalline formations of a wide spectrum of drugs, vitamins, and other assorted biochemicals. For instance, the foregrounds in Figures 3 and 4 are exposures of ascorbic acid (Vitamin C) and chloramphenicol (an antibiotic) respectively, recrystallized after sandwiching the pure biochemical between a microscope coverslip and slide and heating until melted. In some instances, the chemicals recrystallize rapidly within a few minutes. However, many take weeks or even months to completely recrystallize. The composition of the foreground in each photomicrograph is discussed in the respective figure legends.
|
|
Generally, after the selected foreground is exposed, the exposed area is carefully masked by placing a black card over a large enough area of the field lens to completely stop any additional light from reaching the exposed portion of the film. Next, a second exposure is made usually either with Xanthan gum mountain exposure, stretched polyethylene, or through a blue filter in the brightfield mode (See Figures 3-12). In some instances, where a black sky is desired, no filter is used (see Fig. 6 which illustrates a photomicrograph entitled Liquid Crystal Land).
|
|
The field diaphragm image is the next exposure with either a yellow or a red filter inserted into the light pathway. After this exposure is taken, the decentered condenser is left in place and the field diaphragm is opened fully. Next, a microscope slide with a solution of polybenzyl-1-glutamate spherulites is brought into focus, an area devoid of spherulites is placed over the previously exposed field diaphragm image, and an exposure is taken in the polarising mode. This insures that stars do not become imaged in the center of the sun or moon. Careful masking of previously exposed areas (mainly the mountains and foreground) is essential, many times with selectively cut masks, as the exceedingly long exposure times necessary to image the spherulites will create a bleached area (burned) in the mountains or the foreground.
|
|
In Figure 4, the ocean was created by placing a blue filter with a selective mask in the light path (at the field lens) and carefully defocusing the microscope until an ocean skyline effect was obtained. The field diaphragm image was then moved to coincide with the skyline and recorded with a partial mask to resemble a rising sun. The field diaphragm image was subsequently moved to a slightly lower portion of the film and, with careful attention to alignment with the first field diaphragm exposure, exposed after placing a diffraction grating in the light path between the substage condenser and the objective. The diffraction grating was constructed using Polachrome HC 35 mm instant colour transparency film exposed to an extremely bright light to generate a very fine series of lines. This yields a shift in the wavelengths recorded on film to longer (more red) wavelengths and spreads out the image to resemble a reflection of the sun on the water.
|
|
The Many Faces of Ubiquitous Vitamin C
Our various photomicrograph portfolios are each constructed around a central theme as described for the Microscapes collection in detail above. Additional portfolios include a vitamin collection, an antibiotic collection, as well as selected collections of other chemicals and biochemicals.

|
|
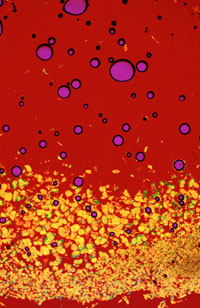
However, probably the most unusual and visually exciting collection is our vitamin collection (Figures 13-20 are selections from this group). A sub-portfolio from the vitamin collection has been named The Many Faces of Vitamin C due to the fact that many of the wide spectrum of crystalline morphologies displayed by recrystallized ascorbic acid resemble faces in one respect or another. Figure 13 is an example of an unusual morphology which possesses a haunting appearance. The pattern illustrated in Figure 14 was titled Vitamin C-Horse by Steven Rosenbaum of Modern Photography due to its striking resemblance to a sea horse. Likewise, surrealistic faces can be found in Figures 15 and 16. Figure 16, a photomicrograph of an isolated crystallite of ascorbic acid has been titled The Man in the Moon-Greencheese due to its uncanny resemblance to the fictional Man in the Moon on one side of the inner circle and to green Swiss cheese on the other side. Currently, we have about 40 members in the Faces of Vitamin C portfolio and constantly search out additional morphological formations from recrystallized ascorbic acid samples.
|
|
The needle-like morphology demonstrated in many recrystallized samples of ascorbic acid can be further enhanced in appearance by the addition of a 530 nanometer retardation plate between the sample and the analysing polariser as is illustrated by the photomicrographs in Figure 17 and 18. By scanning microscope slides and photographing formations in this manner, we have succeeded in generating a large collection of this unusual type of artwork.

|
|
Additional members of The Vitamin Collection are shown in Figures 19 and 20. Niacin, a water soluble member of the B family, when recrystallized from the melt, yields a highly unusual, but beautiful, crystalline morphology as is depicted in Figure 19. Figure 20 is a photomicrograph of Thiamine (Vitamin B1,) partially recrystallized from the melt, imaged with a 530 nanometer retardation plate inserted. It strongly resembles a boiling solution with chips at the bottom. These are only a small sampling from The Vitamin Collection intended to illustrate the inherent beauty to be found in polarized light photomicrographs of recrystallized vitamins.

|
|
Conclusion
By coupling a well-developed photographic methodology to a creative imagination, there is virtually no limit to the types of photomicrographic artwork possible with the light microscope. Here we have demonstrated a few examples of the wide spectrum of possibilities available to the microscopist who is willing to take the time and effort necessary to compose micro-art.

|
|
Experimental Methods
All photomicrographs were composed using a Nikon Optiphot-pol polarizing light microscope in either the brightfield or polarizing mode. A UFX-II digitally controlled exposure monitor measures light intensity through a 30 per cent central portion of the viewfield by employing a photomultiplier and calculates exposure time based on this reading. Illumination is provided by a tungsten-halide 12 volt bulb operating at 8.5-9 volts. Images were recorded on Fujichrome 64 T, a 3200 Kelvin colour-balanced (tungsten) transparency film operating at approximately ISO 64. Exposures were usually made at 1 to 3 f-stops below the recommended exposure of the UFX-II system and Kodak E-6 processing (done in-house) was extended 25-50 per cent in the first developer. Slight modifications were made to the chemical composition of the first colour developers to enhance contrast and colour saturation.
|
|
Acknowledgements
The authors wish to thank Kaye Merchant for her enthusiasm and inspiration during the early days of this study. In addition, we would like to acknowledge the assistance of Tom Fellers (FSU Department of Physics) for advice concerning photographic methods and David Van Winkle (FSU Department of Physics) for advice on liquid crystal behavior. This work was supported, in part, by the NIH.
References
1. Rill, R.L., Hilliard, P.R., and Levy, G. C. (1983): Spontaneous Ordering of DNA. Effects of intermolecular interactions on DNA motional Dynamics monitored by 13 C and 31 P Nuclear Magnetic Resonance spectroscopy. J. Biol. Chem. 258, 250-256.
2. Rill, R.L. (1986): Liquid crystalline phases in concentrated aqueous solutions of NA+ DNA. Proc. Natl. Acad. Sci. USA, 83, 342-346.
3. Strzelecka, T.E. and Rill, R. L. (1987): Solid-State 31 P NMR Studies of DNA Liquid Crystalline Phases. The Isotropic to Cholesteric Transition. J. Am. Chem. Soc. 109, 4513-4518.
4. Strzelecka, T. E., Davidson, M.W., and Rill, R.L. (1988): Multiple liquid crystalline phases of DNA at high concentrations. Nature 331, 457-460.
5. Microscopy and Analysis, November 1988, Issue 8. Note: Additional information relating to the microscopy of liquid crystals can be obtained from: The Microscopy of Liquid Crystals by Norman H. Hartshorne. Published by The McCrone Research Institute Inc., 2820 S. Michigan Ave., Chicago, Illinois 60616 USA (Telephone 302-842-7105).
6. The authors collectively have won prizes in the following competitions:
Nikon Small World Contest, 1987, held by Nikon Inc. Instrument Group, 623 Stewart Avenue, Garden City, New York 11530. Prizes: 6th, 9th, 2 honorable mentions.
Photographic Magazine Monthly Contest, 1987, Held by Peterson's Photographic Magazine, 8490 Sunset Boulevard, Los Angeles, California 90069. Prizes: May, 1987: Honorable mention, July, 1987: Honorable mention.
Polaroid International Instant Photomicrography Competition, 1987, held by the Polaroid Corporation, 575 Technology Square, 9P Cambridge, Massachusetts 02139. Prize: 3rd place.
Olympus Visionage International Photography Contest, 1988, held by the Olympus Optical Co., Ltd., San-Ei Bldg.,22-2 Nishi-Shinjuku, 1-Chome, Shinjuku-Ku, Tokyo 163-19, Japan. Prize: Merit Award.
American Society of Clinical Pathology 1988 Medical Photography Competition Sponsored by Nikon, Inc., held by the American Society of Clinical Pathologists, 2100 West Harrison Street, Chicago, Illinois 60612. Prize: 1st in the Micro Division.
Nikon Small World Contest, 1988, held by Nikon Inc. Instrument Group, 623 Stewart Avenue, Garden City, New York 11530. Prizes: 7th and Honorable Mention.
Polaroid International Instant Photomicrography Competition, 1988, held by the Polaroid Corporation, 575 Technology Square, 9P Cambridge, Massachusetts 02139. Prizes: 1st and Honorable Mention in colour transparency category, and overall best of competition grand prize.
About the Authors
Michael W. Davidson is a research associate in the Institute of Molecular Biophysics at The Florida State University. His main research interests are: photomicroscopy, liquid crystalline DNA, the packaging of DNA into virus capsids, synthetic heterocyclic chemistry, and the interaction of small molecules with DNA.
Randolph L. Rill is a Professor of Chemistry and holds a joint appointment with the Institute of Molecular Biophysics at The Florida State University. His main research interests (in addition of photomicroscopy) are: liquid crystalline DNA, and the packaging of DNA in nucleosomes and chromosomes.