Alexander Jablonski
(1898-1980)

Alexander Jablonski, who would one day come to be known as the father of fluorescence spectroscopy, was born on February 26, 1898 in Voskresenovka, Ukraine. He began his formal journey into the field of atomic physics in 1916 at the University of Kharkov, but was soon delayed due to his service in the military. In 1918, when Poland once again became an independent state after over 120 years of occupation, Jablonski moved to Warsaw and entered the university there to continue his education. He was, however, forced to postpone his study of physics for a time in 1920 when he again undertook military service, this time in the Polish-Bolshevik war.
Jablonski returned to the University of Warsaw in 1921 where studied under Stefan Pienkowski. He also began playing first violin at the Warsaw Opera, an activity he continued for five years before relinquishing it in order to more fully concentrate on scientific endeavors. Despite the various interruptions he had endured, Jablonski received his doctorate in 1930. His doctoral dissertation, entitled "On the influence of the change of wavelengths of excitation light on the fluorescence spectra", concerned what would become the primary focus of his professional career.
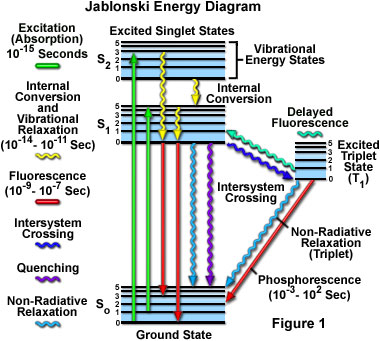
Following the bestowal of his well-deserved degree, Jablonski remained at the university, continuing both theoretical and experimental work in the Department of Experimental Physics. There he closely examined the effects of pressure on atomic spectral lines in gases, but was particularly interested in the polarization of photoluminescence in solutions. In order to explain experimental evidence gained in the field, Jablonski differentiated the transition moments in absorption and in emission, as well as analyzed an assortment of factors responsible for the depolarization of luminescence. His work resulted in his introduction of what is now known as a Jablonski Energy Diagram, a tool that can be used to explain the kinetics and spectra of fluorescence, phosphorescence, and delayed fluorescence.
Jablonski's scientific efforts were halted once again when Germany invaded Poland and World War II began. He served as a member of the Polish Army from 1939 to 1945 and was taken prisoner for a time, first by the German Army and then by the Soviet Army. Jablonski returned to Poland in 1946 to a country completely crippled by the war. In the difficult postwar years, he was elected to chair the Department of Physics at the new Nicholas Copernicus University in Torun. Despite the adverse circumstances in which it was organized, the department under Jablonski's direction became an important center for scientific inquiry into atomic and molecular physics. Jablonski officially retired from the university in 1968, but continued in his private research for many years before his death on September 9, 1980.
Jablonski diagrams (see Figure 1) are often used as a starting point for discussions regarding the absorption and emission of light. A typical Jablonski diagram illustrates a singlet ground electronic state, as well as a singlet first, and sometimes a second, electronic excited state. At each energy level, fluorophores can exist in a number of vibrational energy levels, which are represented by multiple horizontal lines drawn for each electronic state. Transitions between states are depicted by vertical lines that traverse the region between the ground and excited states.
BACK TO FLUORESCENCE INTRODUCTION
