Polarized Light Digital Image Gallery
Pyridoxine (Vitamin B-6)
As one of the central vitamins in the B-complex, pyridoxine (better known as vitamin B-6) is a water-soluble nutrient that is vital as a precursor to a coenzyme involved in transaminations, decarboxylations, racemizations, and numerous modifications to amino acid side chains. In humans, pyridoxine promotes the normal functioning of the brain, blood formation, body fluid balance, and assists in the metabolism of carbohydrates, proteins, and fats.
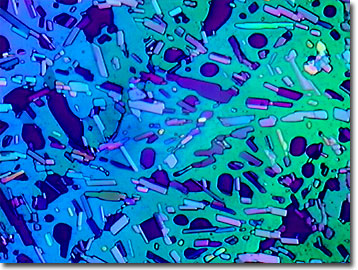
Pyridoxine was discovered in the 1930s as the result of a series of nutritional investigations of rats fed vitamin-free diets. The original compound that was isolated is pyridoxine, named due to its structural similarity with pyridine, but possessing an additional hydroxymethyl group in the para position. In the body, however, the parahydroxymethyl moiety is oxidized to an aldehyde and the similar group in the meta position is phosphorylated, resulting in the biologically active pyridoxal phosphate. Common natural sources of pyridoxine include bananas, carrots, nuts, rice, fish, soybeans, and wheat germ. Symptoms of pyridoxine deficiency are very non-specific and hard to reproduce. The recommended daily allowance (RDA) is 2 milligrams per day and can be obtained through also eating enriched foods such as breads, breakfast cereals, rice, and pastas and dietary supplements. As protein intake increases, the body's need for vitamin B-6 increases.
Commercially synthesized in a hydrochloride formulation, pyridoxine is known to organic chemists as 3,4-pyridinedimethanol, 5-hydroxy-6-methyl hydrochloride or pyridoxol hydrochloride. It has a molecular weight of 205.64 and features 8 carbons, 11 hydrogens, 1 nitrogen, and 3 oxygens atoms plus a hydrogen chloride (HCl) molecule. As a solid, pyridoxine hydrochloride forms white crystals that are soluble in water and melt between 206 and 208 degrees Celsius. It is important to protect this organic chemical from heat, flames, light, and other ignition sources, since decomposition products include hazardous carbon monoxide, carbon dioxide, nitrogen oxides, and hydrochloric acid.
Contributing Authors
Omar Alvarado, Thomas J. Fellers and Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
BACK TO THE POLARIZED LIGHT IMAGE GALLERY
BACK TO THE DIGITAL IMAGE GALLERIES
Questions or comments? Send us an email.
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
Graphics & Web Programming Team
in collaboration with Optical Microscopy at the
National High Magnetic Field Laboratory.
Last Modification Friday, Nov 13, 2015 at 01:19 PM
Access Count Since September 17, 2002: 12790
Visit the website of our partner in introductory microscopy education:
|
|
