Polarized Light Digital Image Gallery
Tacrine (Cognex)
Although tacrine was marketed for many years as a respiratory stimulant, only recently has it been prescribed to treat the symptoms of mild to moderate Alzheimer's disease. Marketed under the trade name Cognex, the reversible cholinesterase inhibitor does not cure or slow the progression of the debilitating disease, but rather allows the patient to improve thinking ability.
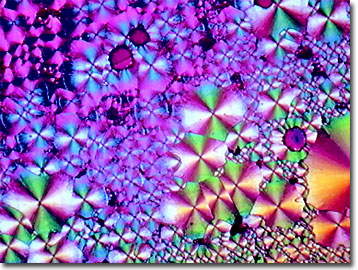
Tacrine is known as 1,2,3,4-tetrahydro-9-acridinamine monochloride monohydrate (or tetrahydroaminoacridine) to biochemists and features 13 carbons, 14 hydrogens, 2 nitrogens plus a molecule of water (2 hydrogens and 1 oxygen) and a molecule of hydrochloric acid (1 hydrogen and 1 chlorine atom) for a molecular weight of 252.74. Tacrine hydrochloride (THA) forms a white solid that is readily water-soluble and soluble in organic solvents. The octahedral crystals of the hydrochloride formulation have a melting point of 283 to 284 degrees Celsius and a bitter taste.
Tacrine slows the breakdown of the chemical messenger acetylcholine, which is apparently accelerated in the cerebral cortices of early Alzheimer's sufferers. There are some indications that HIV/AIDS dementia patients may show significant increases in white blood cell counts and some improvements in movement disorders when taking tacrine. Unwanted side effects of drug therapy with Cognex includes nausea, blurred vision, confusion, shortness of breathe, weakness, and liver problems. Overdoses can cause low blood pressure, extreme weakness, and possible convulsions. In the Ames test, tacrine hydrochloride was mutagenic to bacteria, and as a member of the acridine chemical class, is probably a carcinogen.
Contributing Authors
Omar Alvarado, Thomas J. Fellers and Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
BACK TO THE POLARIZED LIGHT IMAGE GALLERY
BACK TO THE DIGITAL IMAGE GALLERIES
Questions or comments? Send us an email.
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
Graphics & Web Programming Team
in collaboration with Optical Microscopy at the
National High Magnetic Field Laboratory.
Last Modification Friday, Nov 13, 2015 at 01:19 PM
Access Count Since September 17, 2002: 12278
Visit the website of our partner in introductory microscopy education:
|
|
