Polarized Light Digital Image Gallery
Phenylacetic Acid
An aromatic ring compound, phenylacetic acid appears to influence mood and fight depression. Phenylketonuria, a inherited error of catabolism that can cause mental retardation, results in elevated serum levels of the amino acid phenylalanine, some of which is eliminated in the urine and sweat as phenylacetic acid, creating a diagnostic "mousy" body odor.
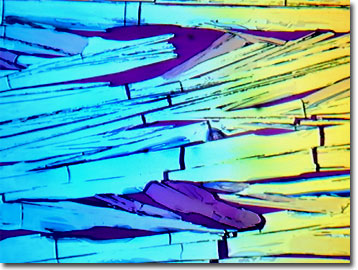
Synthetic phenylacetic acid, also known as benzeneacetic acid, alpha-toluic acid, or 2-phenylacetic acid, forms white crystals with a honey-like odor. With a melting point of 77 degrees Celsius, a boiling point of 265 degrees Celsius, and a molecular weight of 136.1, phenylacetic acid features 8 carbons, 8 hydrogens and 2 oxygen atoms per aromatic ringed molecule. In its pure form, it is classified as toxic and should not be ingested, inhaled, or contacted by the skin. There are also some indications from animal exposure trials that phenylacetic acid may act as a teratogen.
Naturally occurring, phenylacetic acid is derived via enzymatic activity from phenylethylamine. Unlike endorphins, phenylacetic acid can cross from the blood into the brain. Exercise promotes phenylethylamine biosynthesis and increased levels are associated with heightened feelings of physical energy, elevated mood, and sharpened attention. In contrast, depressed people are found to have much lower levels in their body and urine. Structurally similar to amphetamines, it is possible that phenylacetic acid shares the same receptor sites and helps contribute to the well known endorphin-induced "runner's high".
Benzeneacetic acid occurs naturally in Japanese peppermint and rose oils and is used commercially in the production of perfumes and penicillin. In the illicit drug world, phenylacetic acid is often trafficked as a precursor for the bootleg manufacture of methamphetamine, and thus large purchases are monitored by drug enforcement agencies. Synthesizing phenylacetic acid from ethylbenzene, benzyl chloride, benzyl cyanide, mandelic acid, or acetophenone and styrene can be completed in the laboratory or at the industrial level.
Contributing Authors
Omar Alvarado, Thomas J. Fellers and Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
BACK TO THE POLARIZED LIGHT IMAGE GALLERY
BACK TO THE DIGITAL IMAGE GALLERIES
Questions or comments? Send us an email.
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
Graphics & Web Programming Team
in collaboration with Optical Microscopy at the
National High Magnetic Field Laboratory.
Last Modification Friday, Nov 13, 2015 at 01:19 PM
Access Count Since September 17, 2002: 15784
Visit the website of our partner in introductory microscopy education:
|
|
