Polarized Light Digital Image Gallery
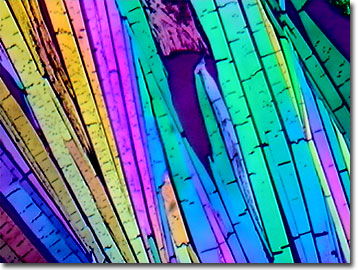
Para-aminobenzoic Acid (PABA)
Para-aminobenzoic acid (PABA), a component of pteroylglutamate, was once considered a vitamin and named vitamin B-X because it serves as a provitamin for some bacteria. Later studies in humans demonstrated that the chemical does not have vitamin activity because humans lack the ability to synthesize folate from PABA.
View a second image of PABA.
This organic chemical was extensively utilized as an effective sunscreen in topical lotions to protect the skin from harmful ultraviolet (UV) radiation associated with sun exposure. However, because of PABA-induced skin irritations, many brands of suntan oil and lotion are marketed as PABA-free. The biochemical is also effective in the treatment of vitiligo, a condition that causes discoloration of the skin, and is included as a nutrient to promote hair growth in many commercial formulations. Natural sources of PABA include bran, eggs, kidney, liver, molasses, wheat germ, brewer's yeast, and yogurt. In humans, PABA is biosynthesized by intestinal bacteria and appears to be vital to their metabolism. There are no reported symptoms arising from a dietary deficiency of PABA, and an overdose may cause nausea, vomiting, and potential liver damage. People taking sulfa antibiotics should not take PABA, because it may reduce their effectiveness.
Known also as vitamin H1 (in the older literature), parminol, and chromotrichia factor, p-aminobenzoic acid features 7 carbons, 7 hydrogens, 1 nitrogen, and 2 oxygen atoms per molecule for a molecular weight of 137.14. Synthesized as white, odorless crystals, para-aminobenzoic acid melts at 187 degrees Celsius and in a 0.5 percent aqueous solution, exhibits a pH of 3.5. Alternative biochemical names for the molecule are 4-amino-benzoic acid, 4-amino benzoesaeure, 1-amino-4-carboxybenzene, and aniline-4-carboxylic acid. Octyl-dimethyl-PABA, a PABA derivative also used in sunscreens, is considered to be estrogenic and an endocrine disrupter. Ethyl-PABA is the effective pain- and itch-relief skin-ointment ingredient known as benzocaine. The chief industrial and chemical uses for PABA are as an intermediate in the production of esters, folic acid, azo dyes, and other organic compounds.
Contributing Authors
Omar Alvarado, Thomas J. Fellers and Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
BACK TO THE POLARIZED LIGHT IMAGE GALLERY
BACK TO THE DIGITAL IMAGE GALLERIES
Questions or comments? Send us an email.
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
Graphics & Web Programming Team
in collaboration with Optical Microscopy at the
National High Magnetic Field Laboratory.
Last Modification Friday, Nov 13, 2015 at 01:19 PM
Access Count Since September 17, 2002: 14617
Visit the website of our partner in introductory microscopy education:
|
|
