Polarized Light Digital Image Gallery
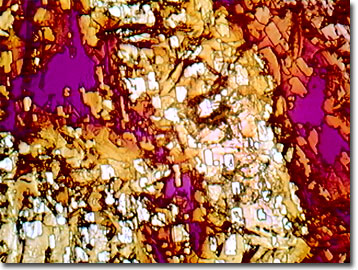
Nicotine
As one of the few liquid alkaloids, nicotine constitutes about 5 percent of a tobacco plant by weight. While nicotine is found throughout the tobacco plant, it occurs in the highest concentrations in the leaves, and ranges from 1 to 8 percent by weight in a typical cigarette.
View a second image of nicotine.
Alkaloids, such as nicotine, are nitrogenous organic compounds that have marked effects on human physiology. Acetylcholine is the natural agonist for the same receptors that bind nicotine, which are particularly concentrated in the central nervous system (brain and spinal cord). As the primary addictive ingredient that acts as a stimulant, nicotine is also marketed over-the-counter as Nicoderm, Nicorette, and ProStep for weaning smokers away from their habits. These products are manufactured as either chewing gum or transdermal patches. Nicotine, evolved by plants to ward off potential herbivores, is a very effective insecticide and aquatic toxicant, and in its purified form, is a deadly poison for some individuals if ingested. However, nicotine is often prescribed to patients suffering strychnine poisoning and tetanus.
In its pure form nicotine is colorless, but when exposed to light or air, it acquires a brown color and gives off a strong tobacco odor. Both nicotine and the genus name for the tobacco plant (Nicotiana) were named for Jean Nicot, a French ambassador to Portugal, who sent tobacco seeds to Paris in 1550. Crude nicotine was known by 1571, and the compound was first purified in 1828. By 1843, the correct molecular formula had been established, and in 1904 it was synthesized in the laboratory. Featuring 10 carbons, 14 hydrogens, and 2 nitrogen atoms per molecule, nicotine is known to biochemists as 3-(1-methyl-2-pyrrolidinyl)pyridine or beta-pyridyl-alpha-N-methylpyrrolidine. It has a boiling point of 247 degrees Celsius and a melting point of -80 degrees Celsius. Interestingly, only the non-polar form of nicotine can enter the cells and blood stream while the polarized, or charged version, simply passes through the body's systems and is eliminated. For this reason, cigarette manufacturers have been well disciplined in including a base in their products in order to remove troublesome protons.
Contributing Authors
Omar Alvarado, Thomas J. Fellers and Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
BACK TO THE POLARIZED LIGHT IMAGE GALLERY
BACK TO THE DIGITAL IMAGE GALLERIES
Questions or comments? Send us an email.
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
Graphics & Web Programming Team
in collaboration with Optical Microscopy at the
National High Magnetic Field Laboratory.
Last Modification Friday, Nov 13, 2015 at 01:19 PM
Access Count Since September 17, 2002: 18307
Visit the website of our partner in introductory microscopy education:
|
|
