Polarized Light Digital Image Gallery
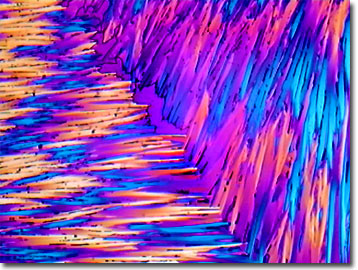
Ibuprofen
Ibuprofen is a nonsteroidal anti-inflammatory drug that is used to treat symptoms caused by arthritis, such as swelling, pain, and stiffness. This drug is widely available without prescription and is marketed under a variety of trade names including Advil, Nuprin, and the original McNeil-PPC formulation, Motrin.
View a second image of ibuprofen.
Ibuprofen is also often used to reduce fever (anti-pyretic), and many people take it as a painkiller (analgesic), although it has not been demonstrated to have remarkable effects on headaches. As with aspirin and paracetomol, two other pain-killing pharmaceuticals, ibuprofen features a six-membered ring structure, which avoids the polar environment of water. In addition, a comparatively small appended group of atoms can assist the drug in bonding to part of the receptor molecule, usually one of the prostaglandins. Prostaglandins are a class of biochemicals that cause inflammation of tissues, leading to pain. Unlike acetaminophen with three polar groups, ibuprofen only has one polar group, the carboxylic acid functional group, making this over-the-counter painkiller somewhat soluble in water and soluble in organic solvents.
To an organic chemist, ibuprofen is a white powder known also as (+/-)-2-(p-isobutylphenyl) propionic acid and has a molecular weight of 206.29, a melting point of about 76 degrees Celsius, and two isomers (R and S). Unlike its predecessor aspirin, ibuprofen is not as likely to create serious gastrointestinal side effects, such as stomach ulcers and internal bleeding. As a peripherally acting analgesic, ibuprofen does not appear to affect any opiate receptors in the brain and has a plasma half-life of 2.2 hours. When filtered by the kidneys and excreted in the urine, approximately 15 percent is unchanged drug, and between 50 and 60 percent are metabolites of ibuprofen. Asthmatic patients must use caution before taking ibuprofen, because of a potential for anaphylactic shock and potentially fatal bronchiospasms. Also, because ibuprofen and the other antipyretic and anti-inflammatory over-the-counter medications treat the symptoms of fever and inflammation, taking these drugs before seeing a physician may mask important diagnostic clues. For some users of ibuprofen, elevated liver enzyme activity (up to 15 percent greater) may suggest liver dysfunction that is only an artifact of the painkiller therapy.
Contributing Authors
Omar Alvarado, Thomas J. Fellers and Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
BACK TO THE POLARIZED LIGHT IMAGE GALLERY
BACK TO THE DIGITAL IMAGE GALLERIES
Questions or comments? Send us an email.
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
Graphics & Web Programming Team
in collaboration with Optical Microscopy at the
National High Magnetic Field Laboratory.
Last Modification Friday, Nov 13, 2015 at 01:19 PM
Access Count Since September 17, 2002: 31022
Visit the website of our partner in introductory microscopy education:
|
|
