Sources of Visible Light
Visible light comprises only a tiny portion of the entire electromagnetic spectrum of radiation. The wavelengths that the human eye can typically visualize lie between 400 and 700 nanometers in length, as illustrated in Figure 1. However, rather than exhibiting a single wavelength, visible light is usually a mixture of wavelengths whose varying composition is a function of the light source from which it is emitted.
During day-to-day living, most people encounter only a small number of visible light sources. When venturing outside, for instance, the vast majority of the light that can be seen is emitted from the sun, which also emits many other frequencies of radiation that do not fall in the visible range. Inside, however, visible light primarily comes from artificial sources, most often fluorescent or tungsten devices.
|
||||||||||||||||||
Table 1
For each set of wavelengths in the visible spectrum, humans perceive certain colors, the distribution of which is outlined in Table 1. Quantification of color is useful because it makes it simpler to differentiate between different hues and shades. It is possible, however, for many different spectral distributions to produce identical color sensations. A yellow color sensation may be caused by a single wavelength of light, for instance 590 nanometers, or it may be the result of viewing two wavelengths, such as 590 and 600 nanometers. It is also possible to view the color yellow as a narrow distribution involving all wavelengths between 590 and 600 nanometers. The same array of possibilities exists for all colors in the visible spectrum.
White light, however, does not appear in Table 1 because it is composed of a mixture containing all or most of the colors in the visible spectrum. White light is emitted by a variety of sources, such as tungsten lamps, which are frequently labeled incandescent because they radiate light when heated by electrical energy. White light may also come from a fluorescent source, in which the light is generated as a result of electrical current traveling through a charged gas. The greatest source of white light, however, is the sun.
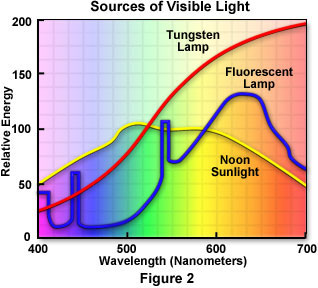
Spectral distribution curves demonstrating the relative amounts of energy versus wavelength for the three most common sources of white light are illustrated in Figure 2. The red spectrum represents the relative energy of tungsten light over the visible spectrum. As is apparent, the energy of tungsten light increases as wavelength increases, which dramatically affects the average color temperature of the resultant light, especially when it is compared to that of natural sunlight and fluorescent light. The yellow spectrum represents what humans see with a natural sunlight spectrum sampled at noon. Under normal circumstances sunlight would have the greatest amount of energy, but the spectrum has been normalized in order to compare it to the other two. The blue spectrum illustrates what is seen with fluorescent light and contains some notable differences from the tungsten and natural sunlight spectra. Several energy peaks are present in the fluorescent light spectrum, which are a result of the superposed line spectrum of mercury vapor in a fluorescent lamp.
| Interactive Java Tutorial | |||||||||||
|
|||||||||||
Since different sources of light exhibit different characteristics, the decision of which type of lighting should be utilized generally depends upon application. For instance, a variety of non-incandescent visible light sources are used for microscopy, indoor, and outdoor lighting. Most of these are based on electronic discharge in a gas such as mercury or the noble gases neon, argon, and xenon. The generation of visible light in these devices relies on the collision of atoms and ions in the gas with the current that is discharged from the electrodes at the ends of the bulbs. This concept is illustrated in Figure 3 by means of a common fluorescent lamp.
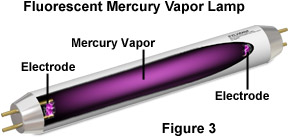
In this example, the glass tube of a fluorescent lamp is coated on the inside with phosphor and the tube is filled with mercury vapor at very low pressure. An electric current is applied to the electrodes at the ends of the tube, producing a stream of electrons. When the electrons collide with mercury atoms, they excite electrons in the atoms to higher energy states. This energy is then released in the form of ultraviolet radiation when the mercury atoms return to the ground state. The ultraviolet radiation energizes the internal phosphor coating causing it to emit the bright white light that is characteristic of fluorescent lights.
A unique feature of non-incandescent visible light sources is that the wavelengths they generate are often concentrated in narrow bands called line spectra. Though they do not produce a continuous spectrum, they are still useful in certain applications. For example, sodium-vapor lamps used in street lighting are an almost exclusively single wavelength source of non-incandescent visible light. These lamps emit a very intense yellow light, over 95 percent of which is composed of 589 nanometer light.
It is possible, however, to design gas-discharge lamps that will emit a moderately continuous spectrum in addition to the line spectra inherent in most of these lamps. The most common method is to coat the inside surface of the tube with phosphor particles, as in the example of common fluorescent lamp. The phosphor particles absorb radiation emitted by the glowing gas and convert it into light ranging in color from red to blue.
Under normal circumstances most people are not able to discern the mixture of a line spectra and a continuous spectrum. However, some objects reflect unusual colors in such an environment, particularly under fluorescent light. This is why clothing purchased in a store illuminated by fluorescent light often appears a slightly different color under natural sunlight or continuous tungsten illumination.
The laser is another important source of visible light, which is becoming increasingly popular for a variety of purposes. Lasers are currently used in applications ranging from compact disk readers to measuring and surgical devices. The word laser is an acronym that stands for Light Amplification by the Stimulated Emission of Radiation. Thus, as their name implies, lasers do not actually generate light, but amplify it.
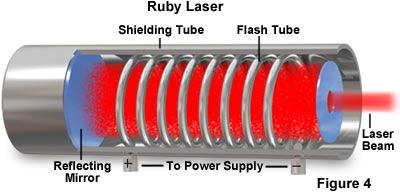
Lasers are unique in that they emit a continuous beam of light made up of a single wavelength that exits in a single phase, commonly termed coherent light. The wavelength of light emitted by a laser depends upon the material from which the laser crystal or gas is composed. The laser illustrated in Figure 4 is a ruby laser that emits red light when atoms in the crystal are excited with a flash tube. The light produced in the gaseous mixture is bounced back and forth between the two mirrored surfaces at the ends of the laser tube, steadily increasing in energy. When a critical threshold is reached, light is emitted from the slightly transparent mirror on one end of the laser tube.
| Interactive Java Tutorial | |||||||||||
|
|||||||||||
In conclusion, there are a wide variety of illumination sources that exist, but humans generally rely on only a few throughout their everyday lives. During daylight hours the sun serves as the main source of illumination outdoors, while fluorescent and tungsten lighting are generally relied upon indoors and during the evening hours. These three primary lighting sources all have different properties and spectral characteristics, but their maximum intensities all fall within the visible light range. Extremely adaptable, the human brain is capable of automatically adjusting to different sources of light and, therefore, the colors of most objects appear almost identical when viewed under any type of illumination.
Contributing Authors
Mortimer Abramowitz - Olympus America, Inc., Two Corporate Center Drive., Melville, New York, 11747.
Shannon H. Neaves and Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
