Observing Mitosis with Fluorescence Microscopy
Cytokinesis
The final stage in the process of cell division is known as cytokinesis, which usually begins during late anaphase or early telophase (before mitosis ends) as the nuclear envelope and nucleoli are reforming and the chromosomes are de-condensing. During cytokinesis, the cytoplasm divides by a process termed cleavage, driven by the tightening of a contractile ring composed of actin and myosin protein subunits. As the ring of cytoskeletal proteins contracts, a cleavage furrow is formed perpendicular to the mitotic spindle and gradually splits the cytoplasm and its contents into two daughter cells.
View a second, third, and fourth fluorescence image of cytokinesis.
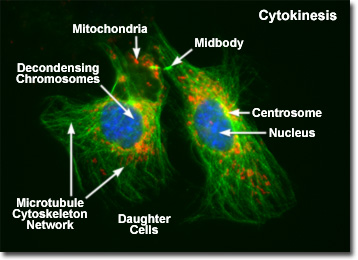
Presented in the digital fluorescence micrograph above is a pair of newly formed daughter rat kangaroo (PtK2) kidney epithelial cells in the late stages of cytokinesis. The chromatin is stained with a blue fluorescent probe (DAPI), while the cytoskeletal microtubule network (mitotic spindle) is stained green (Alexa Fluor 488) and cellular mitochondria are stained with a red dye (MitoTracker Red CMXRos). Note that the mitochondria are becoming interspersed throughout the cytoplasm, the previously condensed chromosomes are forming interphase chromatin, and the mitotic spindle is being redistributed into a cytoskeletal network. A thin bridge between the daughter cells, termed the midbody, is visible under the microscope for several hours before it finally disappears, leaving two completely independent daughter cells.
Orientation of the contractile ring and cleavage furrow, which lie parallel to the metaphase plate and perpendicular to the polar axis of the spindle, is strictly controlled by the mitotic apparatus during cytokinesis. In the microscope, the first visible sign of cleavage in animal cells is an inward folding, or furrowing, of the plasma membrane during late anaphase. A precise orientational relationship between the mitotic spindle and the cleavage furrow ensures that chromatids separated during anaphase will be evenly distributed into the two daughter cells during cell division, guaranteeing that both cells receive identical copies of the genetic material.
In most cases, the cleavage furrow is formed near the equator of the parent cell so that it divides symmetrically into daughter cells of equal size. Positioning of the mitotic spindle axis is thought to be controlled by the astral microtubules emanating from the poles, which interact with motor proteins in the cytoplasm through pushing and pulling forces. In some cases, especially during embryonic development, the spindle is positioned so that the cells divide asymmetrically to produce daughters of unequal size and varying proportions of internal cellular components. The cells produced by programmed asymmetrical division usually proceed through separate developmental phases to form specialized tissues.
The contractile ring that produces cell cleavage is composed of an organized cytoskeletal network that includes actin and bipolar myosin-II filaments working together in a sliding action that mimics muscle contraction. These filaments are bound to the cytoplasmic border of the plasma membrane by a matrix of attachment proteins. During anaphase, components of the ring begin to polymerize on the membrane, and initial contraction of the parallel array occurs in early telophase. The bundle of cytoskeletal fibers contains approximately 20 actin filaments, which slowly disperse to maintain a constant thickness as the ring tightens. At the end of cytokinesis, the contractile ring dissociates, leaving behind only a narrow bridge between the daughter cells (the midbody) filled with actin filaments. When observed in the optical microscope, the midbody reveals a dense matrix of tightly packed polar microtubules remaining from the mitotic spindle.
In higher plant cells, cytokinesis is regulated by the cell wall and occurs by a different mechanism. Plant cells do not divide in the same manner as animal cells through a contractile ring, but assemble a new cell wall between daughter cells instead. Prior to formation of the new wall, an intermediate assembly termed the cell plate is constructed from small membranous vesicles derived from the Golgi complex. The vesicles are guided to the midplane of the mitotic spindle by microtubules derived from polar spindle fibers that are oriented perpendicular to the developing cell plate. In addition, a parallel array of microtubules, termed the phragmoplast, forms in the central region of the cell to guide assembly of the new cell wall. Eventually, the vesicles fuse to form a flattened plate that integrates into the original cell wall to generate two daughter cells.
BACK TO MITOSIS WITH FLUORESCENCE MICROSCOPY