Fluorescence Digital Image Gallery
Normal African Green Monkey Kidney Epithelial Cells (Vero)
|
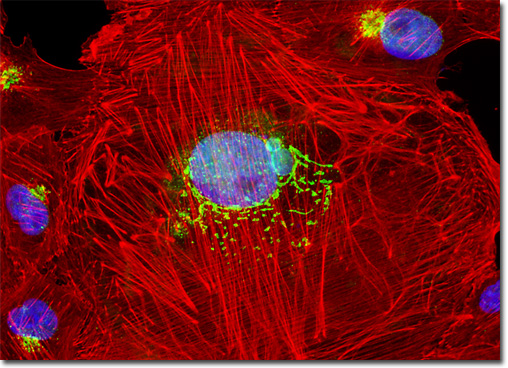
Discovered more than a century ago, lectins are a specialized class of plant proteins that bind to specific carbohydrate groups attached to proteins or residing in cell membranes. Today these proteins are commonly utilized in the laboratory to localize glycoproteins and to detect glycol-conjugates in various applications. The lectin known as wheat germ agglutinin selectively binds to N-acetylglucosamine and N-acetylneuraminic (sialic acid) residues. In solution, wheat germ agglutinin exists as a heterodimer that weighs approximately 38,000 Daltons. Some of the many applications for which wheat germ agglutinin has been utilized include nuclear core studies, investigations of plant hemicelluloses, and examinations of intracellular distribution of altered lysosomal proteins. Conjugates of the lectin are also particularly well suited for staining the Golgi network in fixed cells since a number of proteins and lipids found in the Golgi apparatus are glycosylated. The culture of normal African green monkey kidney epithelial cells (Vero line) presented in the digital image above was labeled with Oregon Green 488 conjugated to the lectin wheat germ agglutinin. The cells were also stained with Alexa Fluor 568 conjugated to phalloidin and DAPI, which target filamentous actin and nuclear DNA, respectively. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles. |
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
|