Fluorescence Digital Image Gallery
Human Bone Osteosarcoma Cells (U-2 OS)
|
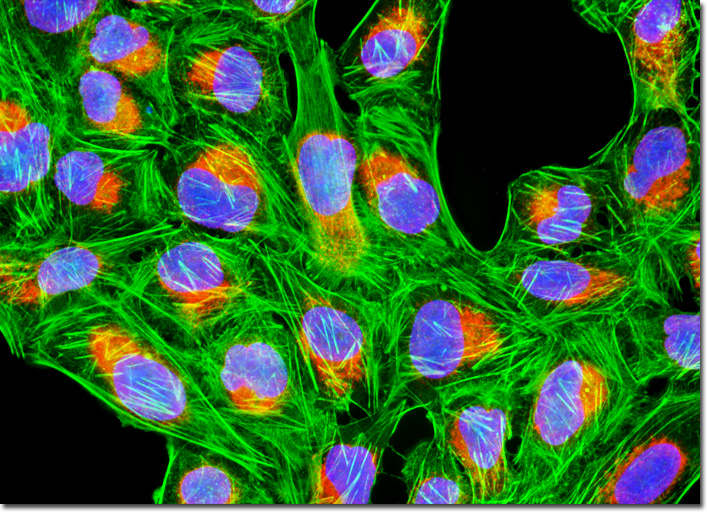
Calreticulin is a major calcium-binding protein that occurs in the membranes of the endoplasmic reticulum (ER) and the sarcoplasmic reticulum (SR), which is a special form of endoplasmic reticulum found in striated muscle fibers. The protein binds calcium ions with low affinity and high capacity, and can release them upon signal. Also known as calregulin, CRP55, CaBP3, calsequestrin-like protein, and Ro/SS-A antigen, calreticulin exhibits the highly conserved sequence Lys-Asp-Glu-Leu (KDEL) at its C-terminus, similar to other proteins resident in the ER. According to recent studies, calreticulin is more multi-functional than previously supposed. Indeed, in addition to its involvement with calcium, the protein may function in chaperoning the folding of glycoproteins, interactions with cellular receptors, RNA-binding, and immune responses. The adherent culture of human osteosarcoma cells displayed in the digital image above was immunofluorescently labeled with primary anti-calreticulin rabbit monoclonal antibodies followed by goat anti-rabbit secondary antibodies conjugated to Cy3, targeting the endoplasmic reticulum. In addition, the cells were stained with Alexa Fluor 488 conjugated to phalloidin and Hoechst 33342, which bind respectively with filamentous actin and nuclear DNA. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles. |
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
|