Fluorescence Digital Image Gallery
Male Rat Kangaroo Kidney Epithelial Cells (PtK2)
|
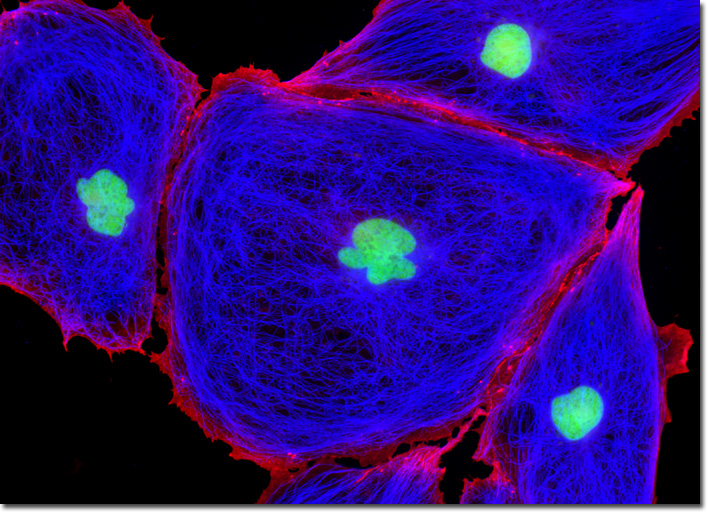
Catenins are a small group of proteins associated with the cytoplasmic domain of uvomorulin, a glycoprotein 120 kiloDaltons in size. Uvomorulin was originally identified in early mouse embryos, where the glycoprotein was found to be involved in the eliciting of antibodies that could block compaction and inhibit the aggregation of teratocarcinoma cells. Thus, catenins, which are generally believed to function in linking to the cytoskeletal network, are often examined in cancer studies. Catenins of the brain have also been of significant interest due to their role in forming synapses, the junctions across which a nerve impulse passes from an axon terminal to a neuron, muscle cell, or gland cell. The technique of double immunofluorescence was employed to simultaneously label the adherent culture of rat kangaroo kidney cells appearing in the digital image above with mouse anti-tubulin and rabbit anti-beta-catenin primary antibodies, followed by goat anti-mouse and anti-rabbit secondary antibodies conjugated to Marina Blue (tubulin) and Rhodamine Red-X (beta-catenin), respectively. The culture was counterstained with SYTOX Green, targeting the DNA present in the cell nucleus. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles. |
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
|