Fluorescence Digital Image Gallery
Indian Muntjac Deer Skin Fibroblast Cells
|
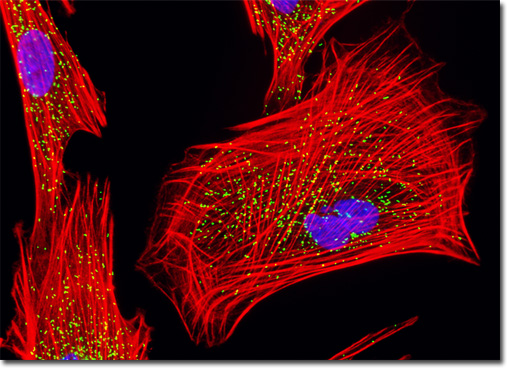
An important membrane polypeptide in peroxisomes, the peroxisomal membrane protein 70 (PMP 70) is generally considered to be a member of the half ATP-binding cassette (ABC) transporter superfamily of proteins. The proteins and enzymes in this superfamily all contain ATP-binding folds and are believed to frequently function in transport across membranes. More specifically, PMP 70 has been associated with the metabolic transport of long chain acyl-CoA across peroxisomal membranes. Also, research suggests that an increase in PMP 70 presence is not involved in the propagation of peroxisomes. However, many of the details regarding the in vivo activities of PMP 70 are still unknown, but many properties are being actively investigated. Studies have demonstrated, for instance, that the isolated protein both homodimerizes and heterodimerizes, but the functional significance of this characteristic has not been adequately established. The peroxisomes present in the Indian Muntjac fibroblast cell culture illustrated in the digital image above were immunofluorescently labeled with Cy2 conjugated to goat secondary antibody fragments directed against rabbit primary antibodies to peroxisomal membrane protein 70 (PMP 70), a major peroxisome membrane polypeptide. In addition, the culture was also labeled for F-actin with Alexa Fluor 568 conjugated to phalloidin, and counterstained for nuclear DNA with DAPI. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles. |
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
|