Fluorescence Digital Image Gallery
Indian Muntjac Deer Skin Fibroblast Cells
|
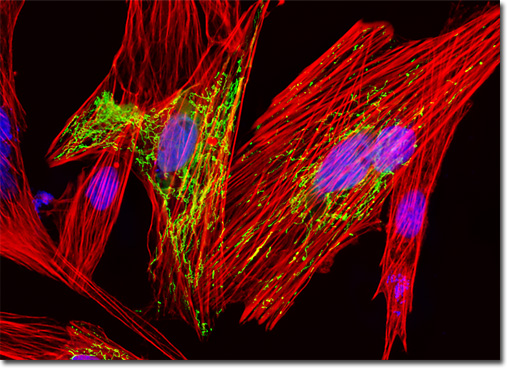
Phalloidin is a bicyclic hexapeptide toxin from the mushroom Amanita phalloides, which is sometimes more commonly known as the death cap. A member of the phallotoxin class of natural products, phalloidin binds specifically to filamentous actin, targeting the junctions between subunits. Since these sites are not typically targeted by other F-actin specific proteins, the majority of the filamentous actin in cell and tissue specimens is accessible to phalloidin or phalloidin conjugates. Exceptions to this rule include the actin-binding protein, nebulin, and the ADF/cofilin group of proteins, both of which compete with phalloidin and other phallotoxins for actin binding sites. The affinity with which phalloidin binds to actin from different species does not vary significantly, unlike the highly selective binding affinity of primary antibodies. The culture of Indian Muntjac deer skin cells presented in the digital image above was labeled with DAPI and Alexa Fluor 568 conjugated to phalloidin, which target DNA in the cell nucleus and the F-actin cytoskeletal network, respectively. In addition, the cells were transfected with a pEYFP-Mitochondria (enhanced yellow fluorescent protein) chimeric plasmid subcellular localization vector. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles. |
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
|