Fluorescence Digital Image Gallery
Human Fetal Lung Fibroblast Cells (MRC-5)
|
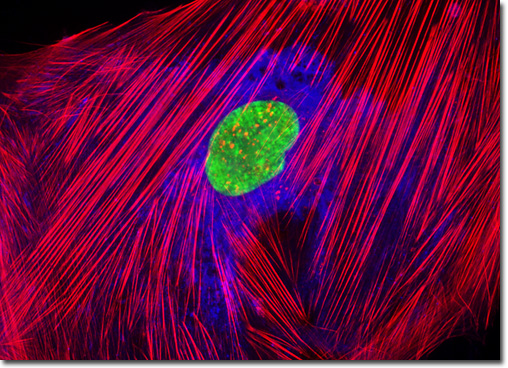
Concanavalin A is one of the most widely utilized of the sugar-binding proteins known as lectins. The protein, which selectively binds to alpha-mannopyranosyl and alpha-glucopyranosyl residues, has, for instance, been utilized for such wide ranging applications as mitotic assays, membrane fluidity investigations, analyses of acrosome reaction incidence in human sperm cells, and hormone receptor studies. Concanavalin A exists as a tetramer of four identical subunits approximately 26,000 Daltons in size each at neutral and alkaline pH levels. Below pH 5.6, however, the lectin dissociates into active dimers. Similar to many other lectins, concanavalin A is derived from the seeds of a leguminous plant, specifically the Jack bean (Canavalia ensiformis). The culture of human fetal lung fibroblast cells presented above was stained with Alexa Fluor 350 conjugated to the lectin concanavalin A, which selectively binds to alpha-mannopyranosyl and alpha-glucopyranosyl residues (primarily in the endoplasmic reticulum). Alexa Fluor 568 conjugated to phalloidin and SYTOX Green were also used to label the culture, targeting filamentous actin and nuclear DNA, respectively. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles. |
© 1995-2025 by Michael W. Davidson and The Florida State University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website means you agree to all of the Legal Terms and Conditions set forth by the owners.
This website is maintained by our
|