Color Temperature
The concept of color temperature is based on the relationship between the temperature of a theoretical standardized material, called a black body radiator, and the energy distribution of its emitted light as the radiator is brought to increasingly higher temperatures. Color temperature is generally expressed in Kelvin (K), which is equivalent to degrees Centigrade (° C) plus 273 degrees. Although a black body radiator does not exist in reality, many metals behave in a manner very similar to the theoretical material.
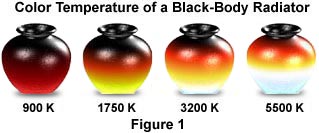
A black metal pot is similar enough to a black body radiator that it can be used as one for discussion purposes, and is, therefore, featured above in Figure 1. The warmest part of a metal pot heated to any temperature is its bottom, and a temperature gradient exists from bottom to top of the radiator. As illustrated, when a metal pot is first heated to a temperature of about 900 K, it begins to glow a dull red. Then, as the temperature is increased to between 1500 and 2000 K, the pot turns into a yellowish, brighter red color. If the temperature is further increased above 3000 K, the color of the pot transforms to a yellow-white, and, finally, at 5000 K and above, a bluish-white color becomes apparent.
| Interactive Java Tutorial | |||||||||||
|
|||||||||||
Light sources are often referred to in terms of their color temperature. In such cases, the color temperature of a light source is defined as the value of the absolute temperature of a black body radiator when the radiator's chromaticity matches that of the light source. However, in the case of fluorescent lamps that can only approximate the chromaticity of a black body, the corrected term "correlated" color temperature is applied through a calculated chromaticity.
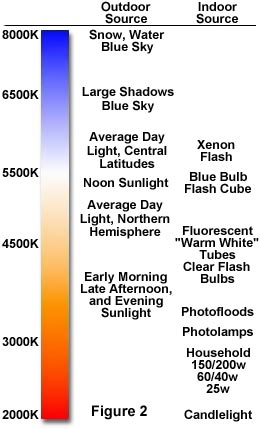
The color temperature chart given below in Figure 2 illustrates the range of colors generated by both artificial indoor sources and natural outdoor sources of lighting. Notice, the lowest temperatures are characterized by the color red and the highest temperatures exhibit shades of blue. This is a point that can easily be confused due to the common tendency of associating red with items that are hot, and blue with items that are cold. It is also important to realize that color temperature values falling below 3500 K are generally considered to be in the tungsten range and neutral colors viewed under this illumination frequently appear redder than they do under the natural daylight of the sun.
The concept of color temperature is especially important in the field of photography, where film emulsions must be carefully balanced to accurately render color using different light sources. For instance, films intended to be used outside in ordinary daylight conditions or with fluorescent or flash lighting are balanced during manufacture for a color temperature of 5500 K. When the proper film is used with a suitable source of light, the color in the resultant image will generally appear natural. However, the 5500 K average daylight color spectrum varies during different parts of the day. Thus, in the early morning and late evening, when the color temperature falls to 5000 K and below, color shifts in the emulsion occur and result in a warmer, redder color rendering.
Most sources of indoor illumination, with the exception of fluorescent lamps, have bulbs that contain some form of tungsten filament. These bulbs emit a wavelength spectrum centered in the 3200 K color temperature region and films color balanced for this type of illumination tend to produce the best results if used properly. Due to their widely different color temperatures, using tungsten balanced films under daylight illumination shifts color tones toward a bluer cast. Contrariwise, using daylight balanced films under tungsten illumination will shift all color tones towards a decidedly yellow cast.
All major film manufacturers have one or several 3200 K films available in 35 mm transparency format, which is the best choice for photomicrographers. Transparency film is preferable to color negative film for several reasons. First, all color negative films are color balanced for 5500 K and must be manipulated during printing to avoid the yellow cast mentioned above. Most photo processors cannot, or will not, produce satisfactory results with photomicrographs on color negative film. Also, the contrast and color saturation in transparency film cannot be equaled by color negative film.
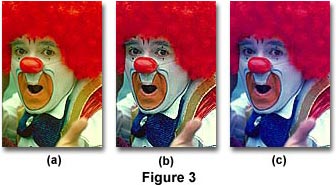
The clown photographs in Figure 3 above further illustrate the proper use of color balance between film emulsions and illumination sources. The clown in Figure 3(b) was photographed under natural sunlight with daylight-balanced (5000 K) Fujichrome Velvia. Using the same film, the clown in Figure 3(a) was photographed indoors under tungsten illumination. Note that all hues are shifted to lower wavelengths and the overall image has a definite yellow cast. The clown in Figure 3(c) was photographed under natural sunlight like the one in 3(b), but this time the film was tungsten-balanced (3200 K) Fujichrome 64T. Under these conditions, the image has an overall blue cast and appears very unnatural, especially when compared to Figure 3(b). Thus, careful coordination of lighting conditions and film emulsion must take place in order for photographers to capture beautiful images that accurately reproduce the actual colors of the subject.
Contributing Authors
Mortimer Abramowitz - Olympus America, Inc., Two Corporate Center Drive., Melville, New York, 11747.
Shannon H. Neaves and Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
