Darkfield Microscopy
The Intel QX3 microscope is designed exclusively for work using brightfield illumination in which the specimen is illuminated either with transmitted or reflected light. This mode of illumination is perfectly adequate for examining transparent stained specimens with transmitted light or for reflected light observation of opaque specimens such as insects, integrated circuits, plants, and other dense objects.
An alternative to standard brightfield illumination, commonly used in optical microscopy, is darkfield illumination, which is a simple and popular method for making unstained objects clearly visible. Many specimens, including living cells and small aquatic organisms such as algae and protozoans, are difficult to visualize with brightfield microscopy because their refractive index is very close to water, the surrounding medium. Other specimens difficult to detect with brightfield transmitted light are diatoms, fibers, hair and fur, mineral thin sections, and small insects. These are ideal candidates for illumination with a darkfield microscope.
Darkfield illumination requires blocking out of the central light that ordinarily passes through and around the specimen, allowing only those rays converging at oblique angles to strike the specimen. This results in a brightly illuminated specimen appearing on a very dark background, which greatly increases contrast and visibility of the specimen.
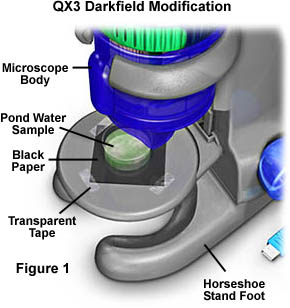
Ordinary laboratory or teaching microscopes are equipped with a substage condenser that can be easily modified for darkfield illumination as described in our microscopy primer. The Intel QX3 microscope can also be modified for darkfield microscopy using the reflected light illuminator that is housed in the microscope body. Figure 1 illustrates such a microscope configuration for darkfield illumination.
In order to prepare the Intel QX3 for darkfield microscopy, a 2-inch square section of black paper is cut and taped over the diffusion filter screen to block stray light passing from the light mixing chamber. Next, the object to be observed in darkfield is placed on top of the black paper and illuminated with the lamp that resides in the microscope body, or with the aid of external oblique illumination from a second source. Choose a paper that is very course and will not reflect light very efficiently. Black construction paper is ideal for this purpose. This form of darkfield illumination does not conform to the classic conditions for either transmitted or reflected darkfield microscopy, but is actually a hybrid method taking advantage of reflected light illumination phenomena of miniature specimens on a dark background.
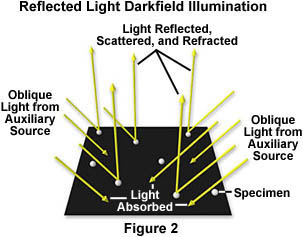
The basic principles of this method are illustrated in Figure 2. Illuminating white light from oblique sources (including the QX3 body lamp) is directed at a microscopic specimen on a dark background. Much of the light strikes the backgound and is absorbed, but some light strikes the specimen and is scattered, refracted, and reflected back into the objective. Through the microscope, the specimen appears brightly illuminated on a dark background as illustrated in Figures 4 through 6. This serves to enhance contrast of specimens that would otherwise be very difficult to detect in brightfield illumination.
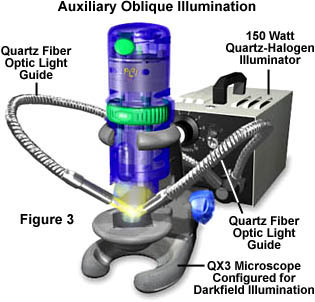
Specimen illumination intensity is a critical factor in reflected darkfield microscopy using the Intel QX3 computer microscope. Without some type of auxiliary oblique illumination, the QX3 body lamp is inadequate in providing the necessary illumination to capture images of the quality displayed in Figures 4 through 6. To assist this lamp in specimen illumination, we suggest the use of external auxiliary light sources similar to the one illustrated in Figure 3. The microscope configuration in Figure 3 capitalizes on additional lighting from a 150-watt quartz halogen illuminator, which funnels light into two quartz fiber optics light guides that can be directed at the specimen. Lamp intensity is controlled by a potentiometer knob on the faceplate of the housing, and this gives the lamp quite a bit of latitiude when adjusting specimen illumination. Lighting angles are also adjustable over a wide range due to the flexibility of the light guides.
If a high-intensity quartz halogen illuminator is not available, there are a variety of high-intensity "Tensor"-style desk lamps commercially available at department stores nationwide. When choosing a desk lamp for use the with QX3, pick the models that have the greatest degree of latitude in positioning the lamp. Models with flexible arms are the ideal choice. A good alternative to the high-intensity desk lamp is the "Snakelight"-style flashlight, which has a flexible arm connecting the battery pack to the lamp. This light source is not as intense as the desk lamps, but it should provide adequate illumination.
To test the microscope configuration, some of the best specimens are the minute organisms found in ponds and lakes. Collect the samples from areas that have decaying vegetation and are rich in these tiny aquatic organisms. A good sampling of pond water will contain an abundance of plant matter, silica from sand and small rocks, and a variety of algae, protozoans, diatoms, rotifers, crustaceans, larve and other creatures. Place a small Petri dish full of the pond water on the stand just below the microscope body and focus the microscope. Look for movement and increase the magnification until the creatures become visible. Two small plastic Petri dishes included with the Intel QX3 microscope are ideal for this experiment.
If clean glass or plastic microscope slides and coverslips are available, it is perferable to place a drop or two of pond water directly on the slide and carefully cover it with a coverslip. This minimizes reflections from the top of the pond water, a problem when viewing microorganisms in a Petri dish. Specimens will appear much sharper and it is possible to record better digital images when the microscope slides and coverslips are used.

Illustrated in Figure 4 is a digital image of a planaria taken with an Intel QX3 microscope operating in reflected darkfield mode using the 60x objective. Note the tiny light-sensitive eye spots that appear as two small dark ellipses on the "head" of the organism. The planaria is winding its way through some sediment in the water and is trying to avoid light from the microscope. There are an abundance of creatures that can be seen and photographed in the pond water including the fly nymph illustrated in Figure 5 below.

These immature flies can maneuver very quickly so you will have to be fast to capture an image of them. Several chemical agents that act to slow down the movements of protozoa and other pond creatures are commercially available. One of the most popular is Boreal Quieting Solution that is available from the educational science supply distributor Boreal Laboratories. A couple of drops of this solution will retard the movements and relax microorganisms such as Paramecium, Euglena and Stentor. A 100 milliliter bottle of Boreal costs about 12 to 15 dollars. Chemical motion retardants are available from most science supply houses, so check catalogs from other manufacturers to find an alternative to Boreal. The science supply resellers also offer culture kits and an "Instant Protozoa Dry Pond Mix" for people who do not have access to fresh water ponds.
As we mentioned above, there are a wide variety of other specimens that are difficult to image with brightfield illumination, but which yield excellent images in darkfield microscopy. Several examples are fibers and hairs, similar to the image of Chinese Goat hair illustrated in Figure 6 below. The image was taken using the highest-power 200x objective, which is the most difficult Intel QX3 microscope objective to focus.

Part of the fun surrounding this incredible computer microscope is the hunt for exciting specimens to image. Small insects such as ants, mites, aphids, and fleas make excellent candidates for imaging in darkfield illumination. Live insects can be immobilized by placing them on double-sided sticky tape and transferring the tape to a microscope slide.
Other interesting specimens include carpet fibers, butterfly and moth wings, sawdust, soap bubbles, ground spices, and many other things found around the house and in the garden. Adding darkfield illumination to the Intel QX3 toolkit expands the capabilities of the microscope and increases the number of interesting specimens that can be digitally imaged.
BACK TO INTEL QX3 SPECIALIZED TECHNIQUES
