Fluorescence Microscopy
Introductory Concepts
When specimens, living or non-living, organic or inorganic, absorb and subsequently re-radiate light, we describe the process as photoluminescence. If the light emission persists for up to a few seconds after the excitation light is withdrawn, the phenomenon is known as phosphorescence. Fluorescence, on the other hand, describes light emission which continues only during the absorption of the excitation light. The time interval between absorption of excitation light and emission of re-radiated light in fluorescence is of extraordinarily short duration, usually less than a millionth of a second.
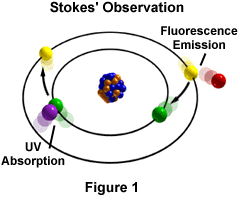
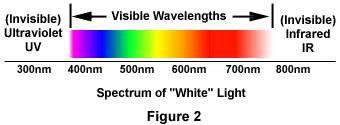
The phenomenon of fluorescence was known by the middle of the nineteenth century. It was Stokes who made the observation that the mineral fluorspar fluoresces when ultraviolet light is directed upon it; he coined the word "fluorescence". Stokes observed that the fluorescing light is in longer wavelengths than those of the excitation light as illustrated in Figure 1 above. In this figure, a photon of ultraviolet radiation (purple) collides with an electron in the atom, exciting and elevating it to a higher energy level. Subsequently, the excited electron relaxes to a lower level and emits light in the form of a lower-energy photon (red) in the visible light region. Figure 2 is a diagrammatic representation of the visible light region of electromagnetic radiation, which covers a wavelength range of approximately 400 to 740 nanometers. Surrounding the visible region is higher energy ultraviolet light and lower energy infrared light.
Fluorescence microscopy is an excellent method of studying material which can be made to fluoresce, either in its natural form (primary or autofluorescence) or when treated with chemicals capable of fluorescing (secondary fluorescence). The fluorescence microscope was devised in the early part of the twentieth century; Koehler, Reichert, and Lehman were among the scientists associated with such development. However, the potential of this instrument was not realized for several decades.
| Interactive Java Tutorial | |||||||||||
|
|||||||||||
Early investigations showed that many specimens (microminerals, crystals, resins, crude drugs, butter, chlorophyll, vitamins, inorganic compounds, etc.) autofluoresce when irradiated with ultraviolet light. However, it was not until the 1930's that Haitinger and others developed the technique of secondary fluorescence--employing fluorochrome stains to stain specific tissue components, bacteria, or other pathogens which do not autofluoresce. These fluorochrome stains, tagged to specific objects, spurred the use of the fluorescence microscope. The instrument's value was significantly enhanced by the 1950's when Coons and Kaplan demonstrated the localization of antigens in tissues that were stained with a fluorescein (the fluorochrome) tagged antibody.
The basic task of the fluorescence microscope is to permit excitation light to irradiate the specimen and then to separate the much weaker re-radiating fluorescent light from the brighter excitation light. Thus, only the emission light reaches the eye or other detector. The resulting fluorescing areas shine against a dark background with sufficient contrast to permit detection. The darker the background of the non-fluorescing material, the more efficient the instrument.
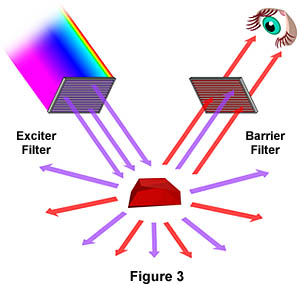
Figure 3 is a graphical representation of what happens when a fluorescing sample is observed with a fluorescence microscope. Ultraviolet (UV) light of a specific wavelength or set of wavelengths is produced by passing light from a UV-emitting source through the exciter filter. The filtered UV light illuminates the specimen, in this case a crystal of fluorspar, which emits fluorescent light when illuminated with ultraviolet light. Visible light emitted from the specimen, red in this case, is then filtered through a barrier filter that does not allow reflected UV light to pass. It should be noted that this is the only mode of microscopy in which the specimen, subsequent to excitation, gives off its own light. The emitted light re-radiates spherically in all directions, regardless of the direction of the exciting light.
Fluorescence microscopy is a rapidly expanding and invaluable tool of investigation. Its advantages are based upon attributes not as readily available in other optical microscopy techniques. The use of fluorochromes has made it possible to identify cells and sub-microscopic cellular components and entities with a high degree of specificity amidst non-fluorescing material. What is more, the fluorescence microscope can reveal the presence of fluorescing material with exquisite sensitivity. An extremely small number of fluorescing molecules (as few as 50 molecules per cubic micron) can be detected. In a given sample, through the use of multiple staining, different probes will reveal the presence of different target molecules. Although the fluorescence microscope cannot provide spatial resolution below the diffraction limit of the respective objects, the presence of fluorescing molecules below such limits is made visible.
Techniques of fluorescence microscopy can be applied to organic material, formerly living material or to living material (with the use of in vitro or in vivo fluorochromes) or to inorganic material (lately, especially in the investigation of contaminants on semiconductor wafers). There are also a burgeoning number of studies using fluorescent probes to monitor rapidly changing physiological ion concentrations (calcium, magnesium, etc.) and pH values in living cells.
There are specimens that fluoresce when irradiated with shorter wavelength light (primary or autofluorescence). Autofluorescence has been found useful in plant studies, coal petrography, sedimentary rock petrology, and in the semiconductor industry. In the study of animal tissues or pathogens, autofluorescence is often either extremely faint or of such non-specificity as to make autofluorescence of minimal use. Of far greater value for such specimens are the fluorochromes (also called fluorophores) which are excited by irradiating light and whose eventual yield of emitted light is of greater intensity. Such fluorescence is called secondary fluorescence.
Fluorochromes are stains, somewhat similar to the better-known tissue stains, which attach themselves to visible or sub-visible organic matter. These fluorochromes, capable of absorbing and then re-radiating light, are often highly specific in their attachment targeting and have significant yield in absorption-emission ratios. This makes them extremely valuable in biological application. The growth in the use of fluorescent microscopes is closely linked to the development of hundreds of fluorochromes with known intensity curves of excitation and emission and well-understood biological structure targets.
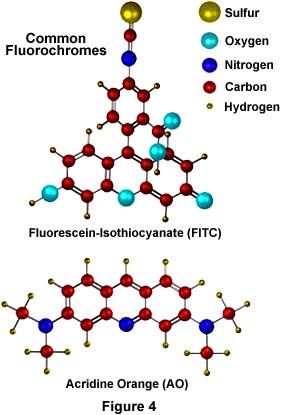
Illustrated in Figure 4 are two of the most commonly used fluorochromes in fluorescence microscopy. Fluorescein-isothiocyanate (FITC), the upper molecule in Figure 4, is a xanthine derivative with a highly reactive isothiocyanate moiety that is useful for fluorescent labeling of proteins and has been widely used in the fluorescent antibody technique for rapid identification of pathogens. Below the FITC molecule is another fluorochrome, acridine orange (AO) whose DNA-binding and fluorescent properties have been thoroughly studied. AO interacts with DNA in a highly specific manner by intercalation of the acridine nucleus between successive DNA base pairs to actually increase the effective length of the DNA molecule. This type of binding allows AO to be used as a fluorescent probe for cellular DNA. These molecules (and other similar fluorochromes) are highly conjugated aromatic systems whose electronic properties allow for high fluorescence quantum yields.
When deciding which label to use for fluorescence microscopy, it should be kept in mind that the chosen fluorochrome should have a high likelihood of absorbing the exciting light and should remain attached to the target molecules; the fluorochrome should also be capable of providing a satisfactory yield of emitted fluorescence light. There are hundreds of fluorochromes that have been found useful for microscopy and many of them are listed in our fluorochrome information tables contained in a separate section of the primer.
Contributing Authors
Mortimer Abramowitz - Olympus America, Inc., Two Corporate Center Drive., Melville, New York, 11747.
Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
BACK TO FLUORESCENCE MICROSCOPY
