Fluorescence Digital Image Gallery
Madin-Darby Ovine Kidney Epithelial Cells (MDOK Line)
The Madin-Darby ovine kidney (MDOK) cell line was derived from the renal tissue of a male sheep. The line exhibits typical epithelial morphology and is susceptible to several viruses including vesicular stomatitis (Indiana and New Jersey strains), infectious bovine rhinotracheitis, and sheep bluetongue virus.
Epithelial cells generally exist in the body in sheets covering the organs and other internal and external surfaces that may come into contact with foreign materials. Usually the cells contain a relatively large amount of cytoplasm and a significant quantity of granules. The function of the cells is various, some acting in an absorptive or protective role, while others primarily act as secretory cells.
The kidneys are divided into two primary sections: an outer cortex and an inner medulla. The cortex is typically dark brownish-red in color due to the large amounts of blood it contains, and has a granular appearance due to a proliferation of renal corpuscles. Much paler, however, is the medulla, which only contains small amounts of blood. This portion of the kidney exhibits a number of pyramid-shaped striated regions referred to as the renal pyramids, which are divided from one another by sections of tissue known as the cortical columns. The apices of the pyramids are called renal papillae and each one exhibits a number of openings called the ducts of Bellini. Each apex is also surrounded by a minor calyx, which joins nearby calyces to form a series of major calyces that empty into the area called the renal pelvis, into which urine is released before it enters the ureter.
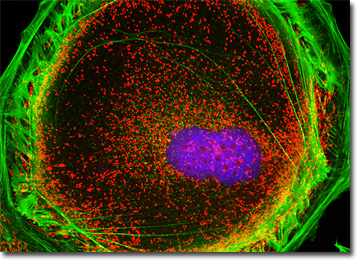
The culture of Madin-Darby ovine kidney (MDOK) cells presented in the digital image above was labeled with MitoTracker Red CMXRos and Alexa Fluor 488 conjugated to phalloidin, targeting the mitochondrial network and filamentous actin, respectively. The culture was counterstained for DNA in the cell nucleus with Hoechst 33258. Images were recorded in grayscale with a QImaging Retiga Fast-EXi camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles.
Additional Fluorescence Images of Madin-Darby Ovine Kidney (MDOK) Cells
Histone and Peroxisome Distribution in MDOK Cells - Nuclear histone proteins were targeted in a culture of Madin-Darby ovine kidney cells with mouse anti-histone (pan) monoclonal antibodies, which were imaged with goat anti-mouse Fab fragments conjugated to Texas Red (labeling the nucleus). The specimen was simultaneously labeled for peroxisomes with Alexa Fluor 488 conjugated to goat secondary antibodies that target rabbit anti-PMP 70 (peroxisomal membrane protein 70). Alexa Fluor 350 conjugated to phalloidin was utilized to counterstain the cytoskeletal F-actin network.
Visualizing the Proximity of Intermediate Filaments and Cytoskeletal Filamentous Actin in Madin-Darby Ovine Kidney Cells - A log phase adherent culture of MDOK cells was fixed and permeabilized with a cocktail of paraformaldehyde, Triton-X100, and glutaraldehyde before being blocked with 10-percent normal goat serum. Subsequent treatment with mouse anti-vimentin monoclonal primary antibodies was followed by goat anti-mouse secondary antibodies (IgG) conjugated to Texas Red-X. In addition, the filamentous actin was labeled with Oregon Green 488 conjugated to phalloidin and the nuclei counterstained with Hoechst 33258.
Madin-Darby Ovine Kidney Cells with MitoTracker Red CMXRos, Alexa Fluor 488, and Hoechst 33258 - The mitochondrial network in a culture of MDOK epithelial cells was labeled with MitoTracker Red CMXRos, a derivative of X-rosamine. In addition, the cells were stained with Alexa Fluor 488 conjugated to phalloidin and Hoechst 33258, targeting filamentous actin and nuclear DNA, respectively.
Distribution of Tubulin and Mitochondria in MDOK Cell Cultures - A culture of sheep kidney cells (MDOK line) was immunofluorescently labeled with primary anti-tubulin mouse monoclonal antibodies followed by goat anti-mouse Fab fragments conjugated to fluorescein. The cells were simultaneously probed for DNA with the ultraviolet-absorbing probe Hoechst 33258, and for the mitochondrial network with MitoTracker Red CMXRos.
Distribution of the Mitochondrial and Filamentous Actin Networks in MDOK Cells - An adherent MDOK cell culture in log phase growth was treated with MitoTracker Red CMXRos for one hour before being fixed and permeabilized. The cells were subsequently blocked with bovine serum albumen and incubated with Alexa Fluor 350 conjugated to phalloidin, followed by counterstaining with SYTOX Green.
Visualizing Histones and the Golgi Complex in Sheep Kidney (MDOK) Cells - The Madin-Darby ovine kidney cell culture featured in this section was fixed, permeabilized, washed, and blocked with 10-percent normal goat serum in phosphate-buffered saline prior to immunofluorescent labeling with rabbit primary antibodies to giantin, a protein resident in the Golgi complex of mammalian cells. The culture was subsequently stained with a mixture of secondary antibodies conjugated to Alexa Fluor 488. In addition, histones were immunofluorescently labeled with primary anti-histone mouse monoclonal antibodies followed by goat anti-mouse Fab heavy and light chain fragments conjugated to Texas Red.
Madin-Darby Ovine Kidney Cells with Concanavalin A - A fixed and permeabilized adherent culture of MDOK cells was treated with a cocktail of concanavalin A conjugated to Texas Red and Alexa Fluor 488 conjugated to phalloidin to label the endoplasmic reticulum and filamentous actin networks, respectively. The nuclei were subsequently counterstained with Hoechst 33258.
Cytokenesis in Growing Cultures of MDOK Cells - Immunofluorescence with mouse anti-alpha-tubulin was employed to visualize distribution of the microtubule network in dividing Madin-Darby ovine kidney epithelial cell cultures. The secondary antibody (goat anti-mouse IgG) was conjugated to Alexa Fluor 568 and mixed with Alexa Fluor 350 conjugated to phalloidin to simultaneously image tubulin and the actin cytoskeleton. Nuclei were counterstained with SYTOX Green.
Cytokeratin Intermediate Filaments in Madin-Darby Ovine Kidney Epithelial Cells - The cytokeratin intermediate filament network in a culture of MDOK cells was visualized by fixing an adherent culture in methanol, followed by treatment with mouse anti-cytokeratin (pan) monoclonal antibodies and goat anti-mouse Fab fragments conjugated to Cy2. Prior to fixation, the cells were exposed to MitoTracker Red CMXRos for one hour, and the culture was subsequently counterstained with Hoechst 33258 to image DNA in the nuclei.
Adherent MDOK Cell Cultures with Alexa Fluor 350, Texas Red, and Alexa Fluor 488 - In a double immunofluorescence experiment, a culture of Madin-Darby ovine kidney cells was fixed, permeabilized, and blocked with 10-percent goat serum before being treated with a cocktail of mouse anti-histones (pan) and rabbit anti-giantin primary antibodies. The target organelles were visualized with goat anti-mouse and anti-rabbit secondary antibodies (IgG) conjugated to Texas Red and Alexa Fluor 488, respectively. The filamentous actin cytoskeletal network was imaged with Alexa Fluor 350 conjugated to phalloidin.
Visualizing the Nucleus, Mitochondria, and Filamentous Actin Network in Madin-Darby Ovine Kidney Cells - A log phase culture of Madin-Darby ovine kidney (MDOK) cells was treated with MitoTracker Red CMXRos, and then washed, and fixed with paraformaldehyde in medium containing serum. After several washes with phosphate-saline buffer, the cells were permeabilized and blocked with bovine serum albumen before labeling with Alexa Fluor 488 conjugated to phalloidin. The nuclei were subsequently counterstained with Hoechst 33258.
Mitochondrial Network Organization by Microtubules in MDOK Cell Cultures - In order to visualize the interrelationship between the microtubule and mitochondrial networks in MDOK cells, a rapidly growing adherent culture was stained with MitoTracker Red CMXRos, fixed, permeabilized, and then treated with mouse primary antibodies to tubulin. The culture was subsequently labeled with goat anti-mouse Fab antibody fragments conjugated to fluorescein and counterstained with Hoechst 33258 (targeting DNA in the nucleus).
Simultaneous Imaging of the Microtubule and Filamentous Actin Networks in Madin-Darby Ovine Kidney Cells - A comparison between the microtubule and actin networks in MDOK cells was conducted by treating a fixed and permeabilized adherent culture with mouse anti-tubulin primary antibodies, followed by a cocktail of goat anti-mouse secondary antibodies conjugated to Alexa Fluor 568 along with phalloidin conjugated to Alexa Fluor 350 (targeting the microtubule and filamentous actin networks, respectively). Nuclei were counterstained with SYTOX Green.
MDOK Epithelial Cell Cultures with Alexa Fluor 350, Alexa Fluor 488, and SYTOX Orange - An adherent monolayer culture of MDOK cells was fixed, permeabilized, and treated with a mixture of concanavalin A conjugated to Alexa Fluor 350 and phalloidin conjugated to Alexa Fluor 488 to target the endoplasmic reticulum and filamentous actin network. After washing, the cells were counterstained with SYTOX Orange to visualize the nuclei.
Madin-Darby Ovine Kidney Epithelial Cells with Texas Red-X, Oregon Green 488, and Hoechst 33258 - In order to label the intermediate filaments in a log phase adherent MDOK culture, the fixed and permeabilized cells were blocked and treated with mouse anti-vimentin (porcine eye lens) primary antibodies followed by goat anti-mouse secondary antibodies (IgG) conjugated to Texas Red-X. Filamentous actin was visualized with phalloidin conjugated to Oregon Green 488, while the nuclei were stained with Hoechst 33258.
Visualizing the Histone, Filamentous Actin, and Golgi Complex Distribution in MDOK Cell Cultures - In a double immunofluorescence experiment similar one described above, a culture of Madin-Darby ovine kidney cells was fixed, permeabilized, and blocked with 10-percent goat serum before being treated with a cocktail of mouse anti-histones (pan) and rabbit anti-giantin primary antibodies. The target organelles were visualized with goat anti-mouse and anti-rabbit secondary antibodies (IgG) conjugated to Texas Red and Alexa Fluor 488, respectively. The filamentous actin cytoskeletal network was imaged with Alexa Fluor 350 conjugated to phalloidin.
Traditional Staining Patterns in MDOK Cell Cultures - The popular dye combination of MitoTracker Red CMXRos, Alexa Fluor 488 conjugated to phalloidin, and Hoechst 33342 were utilized to stain an adherent culture of Madin-Darby ovine kidney epithelial cells. This triple probe regimen labels the tubular mitochondria, filamentous actin network, and DNA in the nucleus.
BACK TO THE CULTURED CELLS FLUORESCENCE GALLERY
BACK TO THE FLUORESCENCE GALLERY
